Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Mergers & Acquisitions
Gilead acquires CymaBay Therapeutics for $4.3 billion
CymaBay’s liver disease asset seladelpar is included in the deal
by Sarah Braner
February 13, 2024
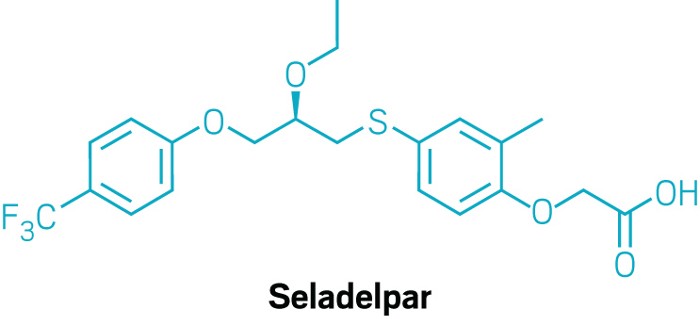
Gilead announced on Monday that it will buy CymaBay Therapeutics for $4.3 billion. The deal includes seladelpar, CymaBay’s small-molecule candidate to treat primary biliary cholangitis.
Primary biliary cholangitis is a chronic condition in which the bile ducts in the liver become inflamed and are eventually destroyed altogether. Seladelpar targets PPARδ, a transcription factor present in multiple liver cells.
CymaBay says that seladelpar reduces inflammation and also reduces the amount of bile created, minimizing its toxic effects on the liver. The US Food and Drug Administration accepted Cymabay’s new drug application for seladelpar, according to a press release from CymaBay that was published on the same day as the deal with Gilead was announced.
Seladelpar originated at Janssen before it was licensed by CymaBay in 2006. back when the firm was called Metabolex. The firm’s name changed in 2013, when it completed a restructuring and jettisoned drug discovery efforts to focus on its three existing product candidates, including seladelpar.
The firm suffered another setback when, in a phase 2b trial for seladelpar in nonalcoholic steatohepatitis, biopsies of liver tissue from patients suggested that the drug may have been causing tissue damage. However, a panel of independent experts concluded that there was no evidence the molecule was at fault.
“The acquisition by Gilead represents a poetic closure to the CymaBay story,” said Andy Hsieh in a research note from William Blair, praising the company’s “resilience and persistence.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter