Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Petrochemicals
Chemistry In Pictures
Chemistry in Pictures: Juicing coal
by Manny I. Fox Morone
June 15, 2023

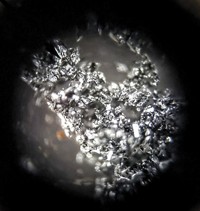
This lustrous carbon coke is what was left of a lump of coal after Christina Thompson was done with it. Thompson, a PhD student at the University of Kentucky, works on ways of converting humble coal to high-performance materials such as carbon fiber and graphite. To make this chunk of carbon coke, her first step was liquefaction, in which she mixed the coal with a petroleum-derived solvent and extracted a solution full of volatile compounds from the bulk material. When Thompson heated the solution at about 400 °C, the volatile chemicals vaporized and escaped from the material, leaving behind these eerie bubbles in the coke as it solidified.
Thompson will finally heat this coke to 2,500 °C to make graphite and test the final material as an anode in lithium-ion batteries. For this project, she used so-called waste coal, which is a by-product of mining that is usually discarded into impoundments because its energy value is too low; these impoundments can pose environmental and safety hazards.
Submitted by Christina Thompson
Do science. Take pictures. Win money. Enter our photo contest here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter