Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Polymer Linkers Play Important Part In Mechanochemical Reactions
Mechanochemistry: Seemingly innocuous molecular groups can boost reaction rate three orders of magnitude
by Lauren K. Wolf
December 24, 2012
| A version of this story appeared in
Volume 90, Issue 52
Self-healing polymers and materials that light up or change color when damaged could be on the horizon, thanks to research on mechanochemical reactions, which transform molecules by mechanical force. So far, though, chemists have focused only on how groups called mechanophores within those materials react under stress or strain.
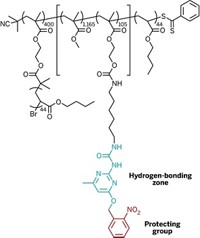
Now, a team led by Stephen L. Craig of Duke University has determined that other seemingly innocuous “filler” components of those materials actually have a significant impact on reactivity. Using single-molecule force spectroscopy, the researchers tugged on strands of polymers interspersed with repeating gem-dihalocyclopropane groups and measured what happened when the groups’ cyclopropane rings opened.
They found that some of the molecular linkers they put between the cyclopropane mechanophores caused the rings to open more easily: When the linkers were polynorbornenes, the reaction proceeded three orders of magnitude faster—that is, required less force—than when the linkers were polybutadienes (Nat. Chem., DOI: 10.1038/nchem.1540).
Using the metaphor of a game of tug-of-war, the Duke researchers say this result is like factoring in the part played by the rope in determining how much force a team needs to exert to topple an opponent.
“This concept establishes a powerful parameter for designing new mechanically active polymers,” says Christopher W. Bielawski, a chemist at the University of Texas, Austin.
Using data modeling, Craig and his group have determined that the polynorbornene linkers act as levers, enabling the polymer strands in which they’re embedded to stretch farther than the polybutadiene linkers do.
The findings, Craig says, have sparked the Duke team to consider how to design polymer scaffolds that react optimally when deformed and to think about incorporating linkers that also transform under force.
In a commentary about the new work, Roman Boulatov, a chemist at the University of Liverpool, in England, says that the Duke group’s measurements help bring chemists closer to being able to predict mechanical reactivity of designer substances, thereby making “mechanochemistry as central to chemical research as chemical kinetics has become.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter