Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Greening Up The Aromatic Finkelstein
Organic Synthesis: Light-driven iodine exchange provides a more straightforward option for preparing versatile iodide reagents
by Stephen K. Ritter
June 29, 2015
| A version of this story appeared in
Volume 93, Issue 26
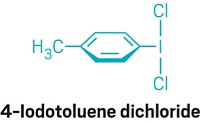
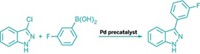
Researchers at McGill University, in Montreal, have reworked the aromatic version of the classic Finkelstein reaction to make it a more straightforward and greener method for preparing iodide reagents (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b03220). Aryl and heteroaryl iodides are sought-after building blocks in organic synthesis for the ease in which they undergo metalation, nucleophilic substitution, and cross-coupling reactions. In addition, radiolabeled versions of the compounds are important for treating hypothyroidism and as contrast agents in medical imaging. But the reactivity that makes the compounds useful makes them difficult to prepare. A leading method is the Finkelstein halogen-exchange reaction, which is carried out by treating an aromatic chloride or bromide with sodium iodide and a copper or nickel diamine catalyst at high temperature. The McGill researchers led by Chao-Jun Li and Zetian Mi realized they could achieve better results without a metal catalyst and at room temperature by using ultraviolet light. By carefully excluding oxygen and adding a dash of elemental iodine, they minimized radical side reactions to prepare high yields of aromatic iodides and diiodides, polycyclic iodides, and vinyl iodides on a gram scale.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter