Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Education
Chemistry In Pictures
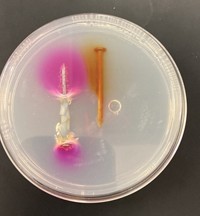
Chemistry in Pictures: Goodbye flowerpot
by Craig Bettenhausen
November 11, 2021

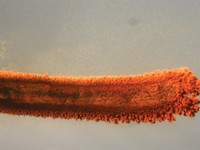
If everyone is careful with safety—goggles, lab coats, fire extinguishers, and a safe distance—the thermite reaction is a knockout chemistry demonstration. Matt Joyner, a biochemistry professor at Pepperdine University, treated his summer research students to a classic presentation of the effect. Iron oxide (rust) powder and aluminum powder are mixed in a terra cotta flowerpot, where the hole at the bottom has been plugged. A heavy iron ring stand holds the pot, and when a fuse of some kind reaches the mixture, well, the photo tells the tale.
All things being equal, oxygen would rather bind to aluminum than to iron, energetically speaking. So iron oxide and aluminum metal should react to form aluminum oxide and iron metal and release a lot of energy in the process, following this chemical reaction: 2Al + Fe2O3 → 2Fe + Al2O3. But the activation barrier for the oxygen to switch metals is high, so mixing the two together cold does nothing. A strip of magnesium, or sometimes a humble sparkler, burns hot enough to get things rolling and then sparks literally fly.
Submitted by Matt Joyner
Do science. Take pictures. Win money. Enter our photo contest here.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter