Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
Superconductor boosts lithium-sulfur battery performance
Adding magnesium diboride to lithium-sulfur electrodes could enable lightweight, high-energy batteries
by Katherine Bourzac
October 29, 2018
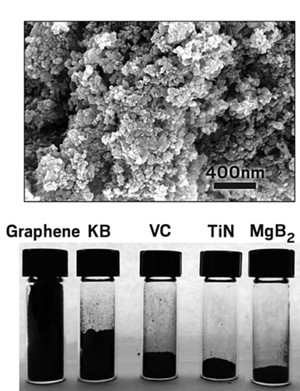
Next-generation batteries based on lithium-sulfur chemistry could store more energy in lighter packages than today’s best lithium-ion batteries. But the intricacies of that chemistry also limit the durability of Li-S batteries. Now researchers have found that they can rein in the chemistry of Li-S cathodes by adding nanoparticles of the superconductor magnesium diboride (Joule 2018, DOI: 10.1016/j.joule.2018.09.024).
Li-S batteries theoretically have a lot of upsides: They could store about five times as much energy by weight as conventional lithium-ion technology; sulfur is abundant, inexpensive, and lightweight; and the element can hold onto a large amount of lithium compared with other electrode materials. But sulfur is fickle, and the chemistry of Li-S batteries is “more fundamentally complicated than lithium-ion battery chemistry,” says Linda Nazar, a battery chemist at the University of Waterloo.
As these batteries charge and discharge, sulfur participates in side reactions, forming a mixture of polysulfides, many of which are soluble in the electrolyte and can move from the cathode to the anode, she explains. That migration means less sulfur remains in the cathode, where it’s needed to take up lithium. These polysulfides also are electrically insulating and form a performance-killing coating on the surface of the anode. As a result of these reactions and migrations, Li-S batteries are not yet efficient or durable enough to provide a viable alternative to lithium-ion batteries.
Unfortunately, chemists’ strategies for preventing these downsides so far have created problems of their own. The typical Li-S cathode starts with a mix of carbon and sulfur. One strategy to mitigate the polysulfide issues is to add sulfur-binding materials to the mix to keep the critical element in place. But these materials also tend to weigh down the cathode or decrease its electrical conductivity.
A few years ago, MgB2 caught Nazar’s attention as a possible way to corral the unruly sulfur. She expected it would, like other additives, bind to polysulfides, but without weighing down the battery or impeding the flow of electrons. That’s because the lightweight compound is a superconductor at low temperatures and highly conductive at room temperature.
Quanquan Pang, who was a grad student in Nazar’s lab and is now a postdoc at Massachusetts Institute of Technology, figured out a way to make MgB2 nanoparticles via a vapor-solid reaction. The synthesis involves combining a boron-carbon mixture with a magnesium pellet and heating it up.
When Nazar’s lab explored the material’s chemistry, they found that both the magnesium and boron on the particles’ surfaces could bind sulfur and prevent it from roving. Also, neither element grabbed any lithium. That’s important because other Li-S electrode additives interact with or bind to lithium, preventing it from circulating and decreasing the efficiency of the battery, Nazar says. The MgB2 nanoparticles, which are about 100 nm wide, also pack well, which means the electrode can be relatively compact, a key consideration given the space constraints of possible applications of such batteries such as use in drones or electric cars.
Advertisement
Finally, the researchers blended the nanoparticles with graphene sheets and sulfur to make a cathode and tested it in a battery, completing the cell with an electrolyte and a lithium-metal anode. The graphene sheets held the cathode together and improved electrical contact with the electrolyte. The electrode’s current output was 14% greater than that of a comparable carbon-based Li-S electrode. The experimental electrode also showed signs of keeping sulfur in line. The battery operated at a high voltage, indicating that pesky polysulfides did not persist. This performance stayed steady as the battery charged and discharged 100 times.
Yuegang Zhang, a battery chemist at Tsinghua University, says the conductive, lightweight MgB2 “has almost all the attributes a sulfur host needs.”
Nazar next plans to make Li-S batteries that can withstand more charging cycles.
CORRECTION: This story was updated Oct. 30, 2018, to correct the name of the graduate student who worked on the MgB2 nanoparticle synthesis.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter