Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Solar Power
New alloy could shrink perovskite solar cell cost
Bilayer electrode made with abundant materials would be easy to print or coat on large-scale
by Prachi Patel, special to C&EN
June 21, 2023
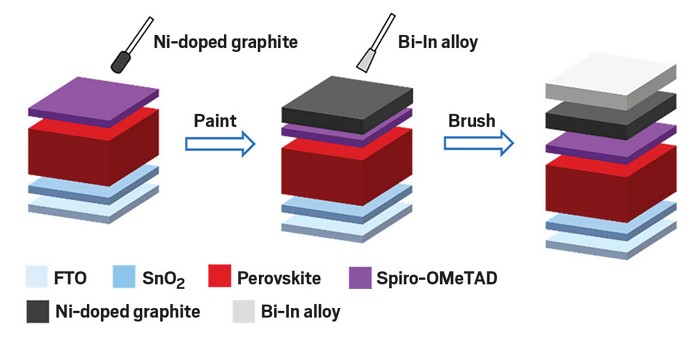
An easy-to-make graphite and metal alloy electrode could be a cheaper alternative to the gold electrodes used in today’s perovskite solar cells (ACS Energy Lett. 2023, DOI: 10.1021/acsenergylett.3c00852).
Perovskite solar cells, which convert sunlight to electricity with greater than 25% efficiency, perform about as well as today’s silicon-based devices. But while perovskites are cheap and can be made simply by depositing precursor solutions, the gold used for the back electrode is expensive, as is making gold films with expensive, time-consuming vacuum-based processes.
Tao Xu of Northern Illinois University, Kai Zhu of the National Renewable Energy Laboratory and their colleagues set out to replace gold with a cheap, abundant material that is easy to process. Gold is used today because it is highly conductive, and because its electron energy levels match those of perovskites, so it can easily extract charges created in the photovoltaic material. “We realized that we could separate these two requirements to two different materials so we don’t have to stick with gold,” Xu says.
For charge extraction, they mixed graphite with nickel to match the perovskite’s electron energy levels. They chose a highly conductive bismuth-indium alloy to transfer charges out of the device. Both the nickel-doped graphite and the metal alloy, given its low melting point of 110 °C, could be printed or blade-coated in ambient conditions.
The team’s analysis shows that for a 1 GW solar plant, their electrode could be manufactured for about 25% the cost of the carbon nanomaterials being considered today to replace gold.
“By potentially reducing both materials cost and infrastructural investment, this technology could enable perovskite solar cells a more attractive option to the multibillion-dollar photovoltaic industry,” says Lin Zhiqun, a materials scientist at the National University of Singapore.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter