Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Water boosts light-driven coupling chemistry
A thin film of organic molecules floating on water can undergo useful photochemical reactions, without needing organic solvents or catalysts
by Mark Peplow, special to C&EN
February 19, 2024
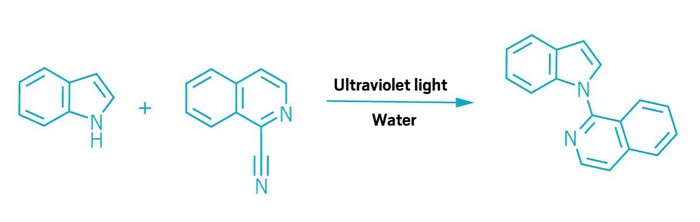
Organic chemists have tended to avoid using water to host reactions, largely because their carbon-based molecules rarely dissolve in the liquid. Now, Burkhard König at the University of Regensburg has turned that insolubility into an advantage. His team has found that a thin film of organic molecules floating on top of water provides the ideal location for light-driven coupling reactions that could be used to modify drug candidates and produce agrochemicals. Interactions between the organic and water layers actually accelerate these reactions, which do not require organic solvents, catalysts, or heat (Science 2024, DOI: 10.1126/science.adl3092).
Chemists already knew that stirring a suspension of insoluble organic molecules in water can sometimes give reactions a boost. These are known as on water reactions (rather than in water), because they take place at the interface where organics and water meet. The reactions run faster for various reasons: the hydrophobic organic molecules are pushed closer together as they shy away from water, for example, while hydrogen bonds formed between the organics and water can also help the reaction along. König’s team has now applied this concept to a range of photochemical reactions. “Water is the ideal solvent for photochemistry; no solvent is more transparent to light,” he says.
The reactions all involve aromatic molecules that can form complexes held together by interactions between their π electrons. When UV light hits the complex, it prompts an electron to move from one molecule to the other, ejecting a leaving group in the process. Hydrogen bonding between water and reactants accelerates this electron transfer step, which ultimately generates radicals that combine to create the product. The method can form various bonds between the reactants, including C–C, C–N, and C–O.
The researchers initially ran the reactions using a layer of oil sitting on water in a vial, but to improve throughput they turned to flow chemistry. Their setup pumps droplets of water, each carrying a film of neat reactants, through a tube that spirals around a light source. The researchers used this flow photochemistry system to couple nitrogen heterocycles to a range of drug molecules. They also showed that it could produce grams of material over several hours. Similar coupling reactions, run in an organic solvent, would normally require a photocatalyst such as an iridium complex.
“The whole setup, I think, is very clever,” says Bao Nguyen, a physical organic chemist at the University of Leeds, who studies organic synthesis on water. “The concept of doing photochemistry in water is not new, but what separates this paper is that it does it without any photocatalyst.”
The method can even work with two solid reactants. Combinations of organic solids sometimes have a lower melting point than their individual components, and may form a eutectic liquid—a type of mixture characterized by this melting behavior—at room temperature. “So you have two white solids and just mix them. They form an oily film on water, and you can start irradiating,” says König. That simplicity has piqued the interest of industry collaborators including BASF, he adds, noting that some of the compounds produced by the coupling reactions could be used as building blocks for crop-protection chemicals, such as herbicides and pesticides.
König hopes that using water in place of organic solvents could also offer a more sustainable approach to making these compounds. However, Nguyen points out that the overall environmental impact of the process will depend on many other factors, including how the starting materials are produced, and how waste products in the water are handled.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter