Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Persistent Pollutants
Tight regulation of Chemours’s GenX chemical proposed in EU
by Cheryl Hogue
March 15, 2019
| A version of this story appeared in
Volume 97, Issue 11

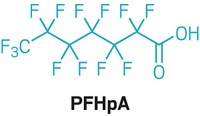
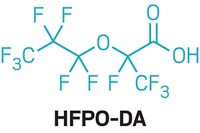
Chemours’s GenX chemical, a polymer-processing aid, would become a candidate for strict regulation in the European Union under a proposal from the Dutch government. GenX is used to produce nonstick coatings and other products. It is an ammonium salt that hydrolyzes into hexafluoropropylene oxide dimer acid (HFPO-DA), which is persistent in the environment and mobile in water. The Dutch proposal says HFPO-DA “adversely impacts human health” at intake levels that could be as low as 21 ng/kg of body weight per day. Animal tests with the chemical have shown harm to the liver, kidneys, blood, and immune system. In contrast, the US Environmental Protection Agency in November proposed a safe daily level for ingestion of 80 ng/kg per day for GenX and HFPO-DA combined. After a public comment period on the Dutch proposal, the European Chemicals Agency (ECHA) will determine whether GenX, HFPO-DA, and related compounds meet criteria for “substances of very high concern” under the EU Registration, Evaluation, Authorisation, and Restriction of Chemicals law. If so, they will become candidates for phaseout in the EU, with their further use allowed only if ECHA authorizes it.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter