Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pollution
Sunscreen chemical kills corals—now scientists know why
The sea creatures metabolize oxybenzone into a toxic compound
by Leigh Krietsch Boerner
May 9, 2022

Oxybenzone, a common active ingredient in sunscreens, is known to damage coral reefs, and some countries and US states have banned swimmers from using sunscreens that contain it. Now a group of scientists led by William Mitch at Stanford University has elucidated the mechanism at work. Corals metabolize oxybenzone to generate a compound that’s toxic to the organisms under sunshine (Science 2022, DOI: 10.1126/science.abn2600). The researchers also found that bleached coral are more vulnerable to the sunscreen chemical.
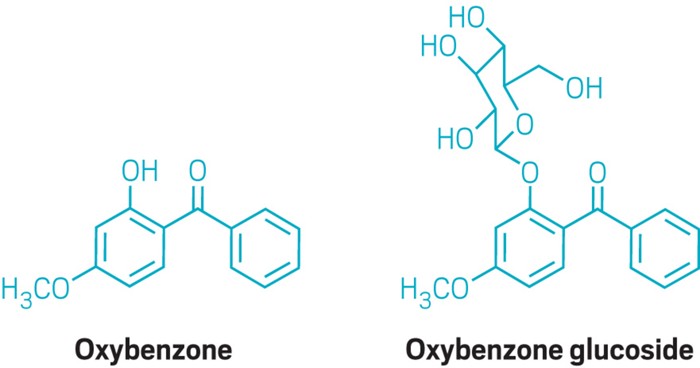
About 11% of sunscreens contain oxybenzone, a UVA and UVB blocker, according to a 2017 report by the US Food and Drug Administration. The group studied the effects of oxybenzone exposure on two model organisms in the coral family—a sea anemone and a mushroom coral. Each day, the group added oxybenzone to simulated seawater in the tanks at levels close to those found in some reef zones. After 17 days, all of the sea anemones were dead. They found that the animals metabolize oxybenzone into phototoxic glucoside conjugates (shown).
Oxybenzone works by absorbing UV light, then releasing the energy as heat. This ability is thanks to the OH group on oxybenzone, says Djordje Vuckovic, graduate student in Mitch’s lab. Once in the high energy state, the OH group is able to wiggle the energy away. But this is no longer possible once the corals metabolize oxybenzone, Vuckovic says. The compound reacts via a glycosylation reaction, where the OH is deprotonated and a glucose molecule adds to the O-. The new oxybenzone glucoside conjugate can still absorb light, but now does not have a way to release the energy as heat. Instead, the excited compound forms reactive oxygen species. “That sets off this radical chain reaction, where eventually you end up causing damage to cells or tissue,” Vuckovic says. The corals “basically convert oxybenzone, a sunscreen, into what is essentially the opposite of a sunscreen, a phototoxin,” he says.
The group’s findings also suggest that corals that have bleached are even more vulnerable to the sunscreen chemical, Vuckovic says. Bleaching happens when corals respond to stresses such as increasing ocean temperatures by expelling the symbiotic algae that live in their cells and are a major source of food and energy for the corals. This exodus causes them to turn white. In the group’s experiments, bleached sea anemones died about 5 times faster than healthy ones. The group found that the anemones’ symbiotic algae soak up the oxybenzone glucoside conjugates, sequestering the phototoxin away from the animals’ cells and protecting them.
Craig Downs, a cell and molecular biologist who directs the nonprofit Haereticus Environmental Laboratory, says the study highlights the relationship between pollution and climate change. “Sunscreen pollution can interact with climate change factors to reduce the resiliency of coral reefs,” Downs says.
“Oxybenzone should not be in coral-safe or reef-friendly sunscreens,” Vuckovic says. Sunscreen compounds with similar structures and mechanisms of action, such as avobenzone, octisalate, and octocrylene, may also damage corals, but more research needs to be done, he says.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter