Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Sustainability
Plastics recycling with microbes and worms is further away than people think
Headlines about plastic-eating organisms belie tough, competitive road to development
by Carmen Drahl
June 15, 2018
| A version of this story appeared in
Volume 96, Issue 25

Credit: Spanish National Research Council/Caption: These polyethylene-eating caterpillars launched a media feeding frenzy, but it's not certain they biochemically degrade the plastic. | These polyethylene-eating caterpillars launched a media feeding frenzy, but it's not certain they biochemically degrade the plastic.
“Could Nature Rid the Planet of Its Plastic Waste?” Over the past few years, worms, bacteria, and enzymes that chow down on polymers have inspired headlines somewhat like this one. The idea that biology may succeed where humans have so far failed is tantalizing.
COVER STORY
The truth about plastic eaters
Except dealing with the staggering amount of plastic waste polluting the earth is more complicated than some coverage has let on. Most reported cases of an enzyme or critter degrading plastic are incomplete and slow. Making these processes faster and more efficient is not trivial. And even if scientists manage to improve polymer biodegradation technologies, they must also bring down the cost to compete in a marketplace that includes chemical recycling methods and virgin monomers—brand-new materials that have never been polymerized.
Media circus notwithstanding, biodepolymerization research is slowly moving forward, and an enzyme-based process to break down one common plastic is making gradual advances to market, which suggests biocatalysis could someday make up a share of—although certainly not the entire—recycling landscape.

Knowing some polymer chemistry can help put glowing headlines in context. Breaking apart polyesters such as poly(ethylene terephthalate) (PET), which was the subject of some studies that made headlines, is relatively easy because these plastics contain the ester bonds that enzymes already degrade in nature. But plastics with an all-carbon backbone, such as the polyethylene in shopping bags or the polystyrene in carryout food containers, are more challenging. News about breaking down those kinds of plastics tends to be greeted with more excitement, but it also merits more skepticism.
In addition, the more neatly a polymer’s molecular chains are arranged, which is measured by a property called crystallinity, the harder they are for enzymes and organisms to break down. Polymers are never 100% crystalline, but some plastics are more crystalline than others. For instance, some studies reported breakdown of flexible PET films, but these films tend to have lower crystallinity, and tend to be easier to chomp on, than plastic bottles.
The very hungry grubs
When an organism appears to be eating plastic, scientists and reporters should look for evidence that the critter is biochemically degrading the polymer rather than simply shredding it. The gold-standard test for confirming the fate of plastic in an organism involves feeding isotopically labeled plastic to animals of interest and tracking whether the labels appear later in monomers or other compounds. Scientists who don’t see this test in a new study are likely to ask for it and to ask more questions.
In 2017, biologist Federica Bertocchini at the Institute of Biomedicine & Biotechnology of Cantabria reported that wax worm caterpillars could break down polyethylene. After their initial observation, they exposed a paste made from mashed-up caterpillars to a sample of polyethylene film, which generated new peaks during a scan with an infrared spectrometer. Bertocchini and her team attributed the peaks to a breakdown product, ethylene glycol, likely generated by a caterpillar enzyme or an enzyme in its gut microbes (Curr. Biol. 2017, DOI: 10.1016/j.cub.2017.02.060). Many outlets, including C&EN, covered the work.
Four months later, however, Till Opatz and his coworkers at Johannes Gutenberg University Mainz published a report disputing the findings (Curr. Biol. 2017, DOI: 10.1016/j.cub.2017.07.004). Bertocchini’s infrared spectra lacked characteristic peaks for ethylene glycol, Opatz argues. He suspected that instead of coming from ethylene glycol, the spectral peaks came from ground-up caterpillar proteins that stuck to the polyethylene film after washing. So his team smeared ground pork, which would also contain proteins, on polyethylene film and obtained a spectrum that Opatz says matched Bertocchini’s. This conclusion doesn’t rule out that caterpillars are capable of biodegrading plastic, but it is a simpler explanation for what Bertocchini’s team observed, Opatz contends.
Bertocchini stands by her work (Curr. Biol. 2017, DOI: 10.1016/j.cub.2017.07.005). “We do need to better check what the by-products of this degradation are,” she says. Funding challenges in Spain have delayed follow-up studies, but her team plans to begin experiments soon.
Some studies do use the gold standard of isotopic labeling to make conclusions about plastic eaters, but scientists may point out other limitations. Jun Yang of Beihang University and his team conducted isotope-labeling studies to determine that a gut bacterium from mealworms consumes polystyrene (Environ. Sci. Technol. 2015, DOI: 10.1021/acs.est.5b02661 and 10.1021/acs.est.5b02663). Work with collaborators such as Wei-Min Wu at Stanford University suggests that the organisms convert about half the polystyrene carbon they ingest to CO2 rather than to styrene monomers.

But the rest of the products—monomers or otherwise—haven’t been identified. And the bacteria don’t completely digest their plastic meals, even after several weeks.
“There appears to be some utilization of polystyrene carbon, but I would still like others to reproduce the results,” says Ramani Narayan, a specialist in biodegradable polymer systems at Michigan State University.
Yang’s team has also reported that Indian meal moth larvae and bacteria within those larvae degrade polyethylene (Environ. Sci. Technol. 2014, DOI: 10.1021/es504038a). But as with Bertocchini’s caterpillar study, no isotope-labeling work was performed. Yang contends it’s possible to confirm biochemical polyethylene breakdown with other techniques, including mass measurements, gel permeation chromatography, and infrared and nuclear magnetic resonance spectroscopy.
Opatz disagrees because contamination could look like degradation products in some of those techniques. Narayan notes that those methods are indirect, and any degradation spotted by them could be attributable to degradation that wasn’t performed by the organism. Moreover, Narayan argues, it’s not enough to show that organisms are biochemically breaking down polymers. Without demonstrating complete degradation, whether to CO2 or recovered monomers, there’s a chance that leftover plastic fragments could wreak havoc on the environment. Microplastics can harm aquatic life that mistakes them for food and transport pollution into the food chain.
It remains to be seen whether enzymes isolated from Yang’s microbes can consume plastic quickly and completely. Yang and his team have sequenced the genome of one of the polyethylene-munching bacterial strains they identified (J. Biotechnol. 2015, DOI: 10.1016/j.jbiotec.2015.02.034), and Yang says work is under way to identify relevant enzymes and to produce greater quantities of them.
Are microbes evolving to eat plastic?
Some bacteria can degrade plastic, which might be nature’s way of adapting to a new food source or just coincidence. We asked sources to weigh in.
“I hope so, but we would definitely need to control them!”
—Federica Bertocchini, Institute of Biomedicine & Biotechnology of Cantabria
“It would only be natural that microbes have and will continue to evolve to consume plastics, since they are increasingly abundant in nature.”
—Richard Gross, Rensselaer Polytechnic Institute
“Whether they’d evolve where there are other things to eat besides plastic is questionable.”
—Emily Flashman, University of Oxford
“This has not yet been demonstrated.”
—Wolfgang Zimmermann, Leipzig University
“We need more examples to answer this question.”
—Kohei Oda, Kyoto Institute of Technology.
Proteins attacking plastic
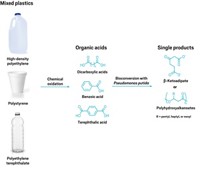
Any enzyme that can break up plastic will be judged by whether it can degrade real-world plastic waste and do so efficiently. In 2016, researchers in Japan tested sludge from a recycling plant and uncovered a microbe that could completely break down films of PET to CO2 and H2O, a feat that was a step above partial degradations reported previously. From that microbe, the scientists plucked two enzymes that degraded PET to its monomers of terephthalic acid and ethylene glycol (Science 2016, DOI: 10.1126/science.aad6359).
Advertisement
Some headlines claimed the microbe could help with recycling, but breakdown took six weeks on a small plastic sample, too slow for immediate applications. Yang, who wasn’t involved with the work, pointed out another caveat of the study in a technical comment in Science: The team in Japan worked with low-crystallinity PET film, which worked better for screening purposes but is relatively easy to degrade compared with the higher-crystallinity PET in water bottles and other common products (2016, DOI: 10.1126/science.aaf8305).

Kohei Oda of Kyoto Institute of Technology, who co-led the original study, says his colleagues set out to screen for microbes that could use PET as a nutrient for growth, regardless of crystallinity (Science 2016, DOI: 10.1126/science.aaf8625). Optimizing enzymes can always come later, he contends. He thinks some news outlets exaggerated the immediate impact of the work, and as a result, he and his coworkers now monitor coverage “very carefully.”
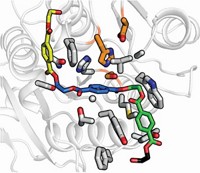
Multiple groups have since solved the X-ray crystal structure of one of the Japanese team’s enzymes, which converts PET to an intermediate called mono-(2-hydroxyethyl) terephthalic acid (Nat. Commun. 2017, DOI: 10.1038/s41467-017-02255-z; Nat. Commun. 2018, DOI: 10.1038/s41467-018-02881-1; Biophys. J. 2018, DOI: 10.1016/j.bpj.2018.02.005; ChemBioChem 2018, DOI: 10.1002/cbic.201800097). But John E. McGeehan of the University of Portsmouth, Gregg T. Beckham of the National Renewable Energy Laboratory, H. Lee Woodcock of the University of South Florida, and their colleagues encountered a pleasant surprise when solving their structure (Proc. Natl. Acad. Sci. USA 2018, DOI: 10.1073/pnas.1718804115). They set out to determine how the PET-degrading enzyme evolved, and in the process, they accidentally made a mutant enzyme that could erode more-highly-crystalline PET than before. Beckham says the current version of the enzyme would still take months to break down a soft-drink bottle. “We’re doing a lot of protein engineering to improve the activity further,” McGeehan says.
Optimization game
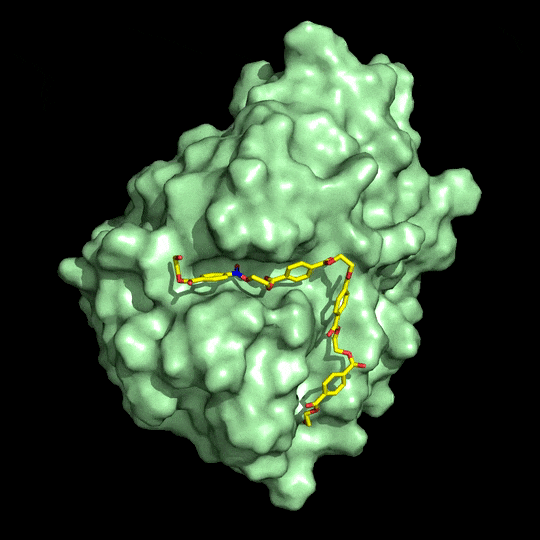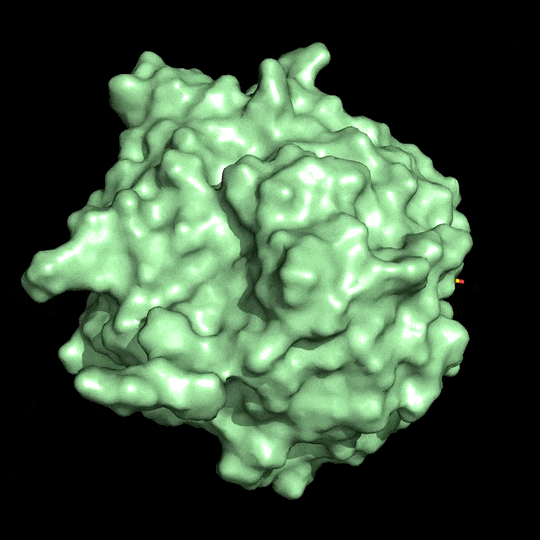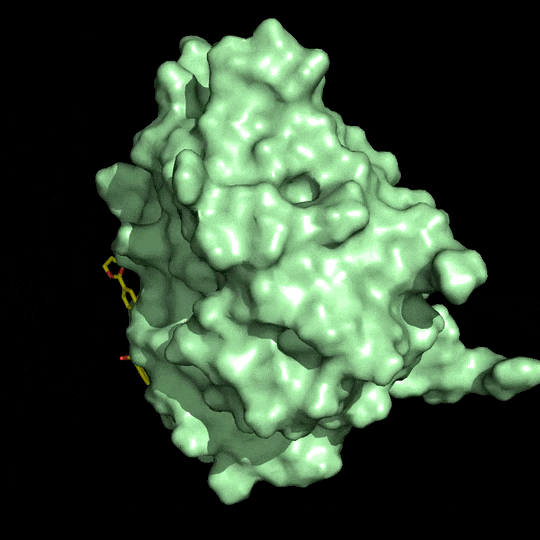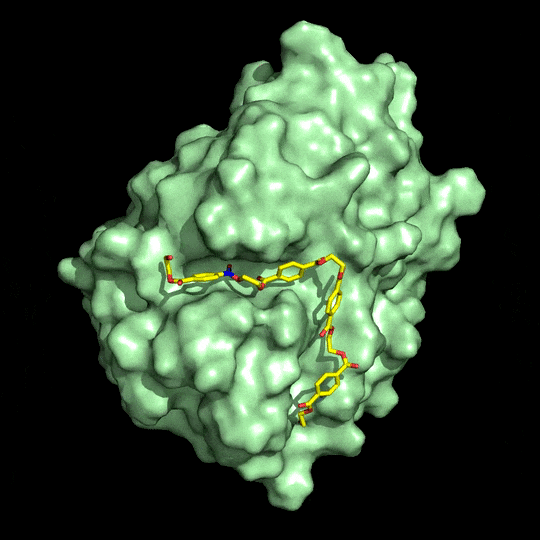
Richard A. Gross has been doing that kind of engineering since 2011. His group at Rensselaer Polytechnic Institute works with cutinases, so named because they degrade cutin, a waxy coating on plants. Many of these enzymes also degrade PET, which, like cutin, is a polyester.
Gross says teams will want to maximize enzyme binding affinity for PET. To boost activity, they’ll also have to make sure the enzyme is stable at or above PET’s glass transition temperature of 75 °C. At the glass transition temperature, polymer chains have higher mobility, which you can think of as having more elbow room, Gross explains, improving an enzyme’s access to the bonds it breaks. And finally, adjustments should be made to block enzyme aggregation, which can reduce performance. Gross’s team showed earlier this year that strategically placed carbohydrate groups largely prevent cutinases from aggregating (Biochemistry 2018, DOI: 10.1021/acs.biochem.7b01189).
Weighing in on McGeehan and Beckham’s work, Emily Flashman, a specialist in enzyme mechanisms at the University of Oxford, adds that to be successful, any biorecycling process must find a productive way to harvest and reuse monomer products. McGeehan says he and his collaborators are “keen to develop” a monomer isolation process, and they are also working to make the enzyme stable at higher temperatures.
The elephant in the room
Even if optimization goes swimmingly, there’s still the matter of cost. Engineered enzymes tend to be more expensive than commodity chemicals, and scaling up a biotechnological process takes yet more investment. “We already have mature, scalable, cost-effective technologies to recycle PET,” such as metal-catalyzed depolymerizations, Narayan says. What’s more, it’s tough to compete with the low price of virgin monomers, he adds. “Nobody wants to face that reality.”
Wolfgang Zimmermann, an expert in biocatalysis for plastics recycling at Leipzig University, is more optimistic. “The plastics manufacturing industry hasn’t been conscious of the waste problem, and this is changing now,” he says. Changing attitudes and changing demands from consumers could tip the balance in favor of recycling methods that use biocatalysts rather than chemical catalysts, if costs can be somewhat competitive, he adds.
The researchers at Carbios aim to capitalize on that sentiment. The French firm says it has optimized an enzyme that depolymerizes 97% of PET starting materials into monomers in 24 hours. “There’s a real gap between what’s been published in the press and what we do,” says spokesperson Benjamin Audebert, referring to news reports about studies in the academic literature. Before exposing PET to its enzyme, Carbios uses a proprietary pretreatment that converts crystalline PET to an amorphous form, facilitating breakdown. Audebert declined to disclose the enzyme Carbios uses, but a company patent mentions bacterial and fungal cutinase and lipase enzymes. Earlier this year, Carbios started scaling up its process to enable treatment of up to 200 kg of PET waste in 24 hours. By year’s end, the goal is to produce a new plastic product, such as a bottle, from recovered monomers and to test the resulting product with corporate partners such as L’Oréal, Audebert says. A 10,000-metric-ton plant is in the works for 2021, he adds. “Our business model is built on the fact that we will be competitive with virgin PET.”

As exciting as PET biorecycling could be, PET is only one plastic, says Nick Wierckx of RWTH Aachen University. The next step would be processes that can degrade plastics with different types of chemical bonds and cocktails of enzymes that could handle mixtures of plastics, he says.
It’s not entirely a hypothetical for Wierckx. He and Zimmermann are two members of a European consortium called P4SB, which aims to convert waste PET and polyurethane to high-value chemical feedstocks (Microb. Biotechnol. 2015, DOI: 10.1111/1751-7915.12312). The consortium’s efforts are largely at the gram scale so far, Wierckx says, but the long-term goal is to create a system similar to what exists for converting biomass to biofuels.
COVER STORY
The truth about plastic eaters
Although plastics biorecycling holds promise, the field has its share of challenges, so the media should be cautious, urges Opatz, the caterpillar skeptic. A step forward shouldn’t be billed as a planet-saving breakthrough. “If you tell the public that this problem has been solved when it hasn’t,” he explains, “people will be waiting for practical solutions, and they’ll be annoyed if in 10 years’ time there’s still polyethylene debris lying around or floating in the oceans. They’ll say, ‘What’s wrong with you scientists? You solved the problem 10 years ago, and we still have this mess.’ ”
At the end of the day, Opatz adds, cavalier coverage “undermines the relationship between science and society.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter