Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
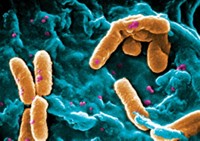
Quat disinfectants are helping during the pandemic. But could they contribute to antibiotic resistance?
Researchers are studying the common disinfectants to determine how they may affect microbes downstream of wastewater treatment plants
by XiaoZhi Lim, special to C&EN
August 30, 2021
| A version of this story appeared in
Volume 99, Issue 31

In October 2020, analytical chemist William Arnold and his team at the University of Minnesota Twin Cities realized they were going to need more freezer space.
Every month for the previous 5 months, the researchers had been going to nearby wastewater treatment plants to collect water samples. But because the university’s COVID-19 restrictions didn’t allow them to work inside their lab to analyze the water, the samples started accumulating in the lab freezer.
The researchers were collecting the water to measure levels of quaternary ammonium compounds (quats). Quats are commonly used in disinfectants, sanitizing wipes, and personal care products. During the COVID-19 pandemic, disinfectant use has shot up, and half the disinfectants approved by the US Environmental Protection Agency to fight SARS-CoV-2, the virus that causes COVID-19, contain quats.
Quat disinfectants are essential for slowing the spread of disease, but the Minnesota researchers are concerned that their increased use could have long-term negative environmental consequences (Environ. Sci. Technol. Lett. 2020, DOI: 10.1021/acs.estlett.0c00437). “They’re biologically active compounds. They’re meant to kill bacteria,” Arnold says. He points out that wastewater treatment plants are biological reactors that depend on microbes to do the hard work of digesting organic matter. A marked rise in quat levels could compromise wastewater treatment processes that rely on this bacterial activity, leading to inadequate treatment of wastewater that then pollutes rivers and other waters downstream.
But Arnold and other researchers have a bigger concern. They worry that an elevated quat presence in the environment could spur the development of antibiotic resistance in bacteria.
Antibiotic resistance is reaching alarming rates across the world. According to data that 82 countries reported to the World Health Organization on over 3 million laboratory-confirmed infections in 2019, 37% of Escherichia coli infections in the bloodstream resisted commonly prescribed cephalosporin antibiotics, and 54% of E. coli urinary tract infections resisted ciprofloxacin treatment. A 2019 United Nations report predicted that by 2050, drug-resistant diseases could cause 10 million deaths annually.
Scientists studying this growing problem have identified wastewater treatment plants as a hot spot for the spread of antibiotic-resistance genes between microbes. The conventional thinking says that microbes mingle with a diverse crowd in the waters at those plants, so they can pick up antibiotic-resistance genes from other organisms that carry them or from genetic material that is floating around. The microbes could also encounter chemicals that stress them out, such as antibiotic molecules, cleaning agents, or heavy metals, and push them to evolve countermeasures to resist those compounds.
Lab studies have shown that exposure to one of the most widely used quats, alkyldimethylbenzylammonium chloride (ADBAC), causes microbial communities to become resistant to some antibiotics. These studies suggest that ADBAC exposure could select for microbes that already have some resistance to antibiotics or favor genetic mutations that confer resistance to antibiotics, says environmental engineer Patrick McNamara of Marquette University.
Because of quats’ widespread use, it is likely that sublethal levels of quats often occur in the environment, where they could pressure bacteria to evolve resistance to them and other antibacterial agents. Quat disinfectants are deployed in a wide variety of places, including homes, hospitals, slaughterhouses, dairy farms, subway trains, and movie theaters. Other than wastewater treatment plants, quats have been detected in rivers, drinking-water sources, estuaries, agricultural fields, and even sediment cores dating back decades.
Researchers like Arnold and McNamara are now trying to better understand the potential for these ever-present quats to drive antibiotic resistance in the environment. They also hope to determine what impact the chemicals’ increased use during the pandemic might have. Others, like Ulas Tezel at Boğaziçi University, are working on methods to degrade quats during wastewater treatment to limit microbes’ exposure to them both in treatment plants and in the wider environment.
Disinfectants containing quats may be necessary in a pandemic, but using them might push bacteria to evolve, perhaps in undesirable ways, Tezel says. Proper stewardship and management of quats, he says, is going to be key to avoiding any unintended consequences.
Quats feature two components that help kill bacteria—a positively charged quaternary ammonium ion and a long hydrophobic tail. The ammonium ion helps a quat molecule stick to negatively charged bacterial membranes, which the greasy tail disrupts.
Researchers have known for a long time that bacteria can acquire resistance to quats. And sometimes when bacteria evolve resistance to one molecular stressor, that mechanism helps them evade or cope with other harmful compounds too. But data on whether bacteria could develop such cross-resistance to antibiotics after bumping into quats remained unclear.


Some of the more definitive data resulted from long-term experiments conducted at the Georgia Institute of Technology. Tezel, who was completing his graduate studies at the time, helped initiate that research, along with environmental engineer Spyros Pavlostathis, who is now retired, and environmental microbiologist Kostas Konstantinidis. The researchers nurtured a microbial community that originated from sediment collected from the Bayou d’Inde, a tributary of the Calcasieu River near Lake Charles, Louisiana. For 3 years, they maintained different versions of this community: two exposed to ADBAC and one not.
When they checked on the microbial communities after 3 years, they found that ADBAC lowered the diversity of microorganisms. The community that was exclusively fed ADBAC comprised mostly members of the Pseudomonas genus (Environ. Sci. Technol. 2013, DOI: 10.1021/es401507k). One species from this genus, Pseudomonas aeruginosa, is an opportunistic pathogen shown to resist multiple antibiotics. The researchers also found elevated levels of resistance to three antibiotics—penicillin G, tetracycline, and ciprofloxacin—in the ADBAC-exposed communities compared with the control community.
A follow-up study led by Konstantinidis found less-straightforward results (Appl. Environ. Microbiol. 2018, DOI: 10.1128/aem.01201-18). When the researchers tested antibiotics on individual species of bacteria from the communities, they found that Achromobacter bacteria that were exposed to ADBAC were more resistant to tetracycline, ciprofloxacin, chloramphenicol, and polymyxin B but more vulnerable to kanamycin, rifampin, and ampicillin than their nonexposed counterparts. Meanwhile, ADBAC-exposed P. aeruginosa exhibited greater resistance to ciprofloxacin, chloramphenicol, and rifampin and lower resistance to tetracycline.
Other researchers have also found mixed results. McNamara, for example, found that a microbial community cultured from water collected from Lake Michigan and exposed to low levels of ADBAC became more resistant to ciprofloxacin but also more vulnerable to ampicillin, streptomycin, and rifampin (Environ. Pollut. 2020, DOI: 10.1016/j.envpol.2019.113472).
These different patterns of resistance suggest to researchers that multiple mechanisms are at work. Konstantinidis and his colleagues identified some mechanisms behind the development of cross-resistance in their 2018 study. By comparing the genomes of P. aeruginosa from the communities with and without ADBAC exposure, the researchers identified a key area where the genomes diverged and found that it coded for four multidrug efflux pumps. These protein complexes allow bacteria to remove toxic compounds such as quats and fluoroquinolone antibiotics, including ciprofloxacin.
Konstantinidis and team also identified a genetic mutation that appeared after ADBAC exposure. They subjected P. aeruginosa to short-term, intense ADBAC treatment and found that the bacteria became more resistant to polymyxin B than control groups. When the researchers examined the exposed bacteria’s genomes, they found changes in a single gene that encoded for a modification on the bacteria’s outer cell membranes. This modification reduces the negative charges on the membranes, which may have reduced the membrane’s attraction to both ADBAC and the positively charged antibiotic, the team proposed.
But just because bacteria acquire resistance to antibiotics after quat exposure in lab studies does not mean they do so in the real world. Even though the laboratory studies show that the possibility exists, the risk of antibiotic resistance developing because of quats in the environment remains low, says Paul DeLeo, a researcher from Integral Consulting, which is funded by companies that manufacture quats.
To determine whether quat exposure could drive antibiotic resistance in real-world bacteria, “we need to know what is the exposure of the microbial communities in the environment,” says Florian Breider of the Swiss Federal Institute of Technology, Lausanne (EPFL). After establishing microbes’ environmental exposure to quats, scientists can then compare those levels with the experimental conditions in lab studies and estimate how likely microbes are to develop antibiotic resistance.
Quantifying quat levels in the environment is not simple. Quats have varying structures. “Even when you say ‘benzalkonium chloride,’ that is not a single compound,” Arnold says. (Benzalkonium chloride is another name for ADBAC.) ADBAC molecules can contain differing alkyl chain lengths, which affect their hydrophobicity and how likely they are to end up in sediment versus water. This structural variation also means that scientists have to look for a range of structures, not just one, when they quantify quats.
Before the pandemic, Arnold’s team documented quats in effluents from wastewater treatment plants in the Minneapolis–Saint Paul metropolitan area. The researchers found quats at levels between 0.4 and 6.6 µg/L, which are dilute compared with those used in disinfectant solutions (Environ. Sci.: Processes Impacts 2020, DOI: 10.1039/c9em00554d). The team also found quats dating back to the 1950s in sediment cores that they collected from Lake Pepin, Lake Winona, and Lake Superior in Minnesota.
Currently, Arnold, McNamara, and a third collaborator, Chris Marshall at Marquette University, are working to measure the levels of quats and antibiotic-resistance genes in selected wastewater treatment plants and water bodies in Minnesota and Wisconsin. One big question for Arnold is the mass balance of quats—How much goes into a plant, and how much comes out? That calculation would help provide an idea of how well quats are broken down in wastewater treatment plants and how much escapes to the wider environment.
McNamara and Marshall are working to quantify the antibiotic-resistance genes and antibiotic-resistant bacteria in the same samples so that they can compare those data with quat levels to see if there are any significant correlations.
Still, the puzzle is missing some pieces. DeLeo notes that the overuse of antibiotics directly affects clinically relevant pathogens, but the use of quat disinfectants does not have straightforward links to the same pathogens. More steps need to occur to connect quats and microorganisms that infect people. “I don’t want to underplay the seriousness of this, but there’s a difference between a pathogen getting antibiotic resistance and a general organism getting antibiotic resistance,” he says. For quat-fueled resistance to be problematic, genetic material coding for antibiotic resistance needs to jump from microbes harmless to humans to pathogens that infect people.

This process, called horizontal gene transfer, is poorly understood. Konstantinidis is no longer studying quats, but he is working to understand horizontal gene transfer, specifically how frequently it occurs in the environment and what factors might increase its probability.
While these scientists worry about quats’ ability to increase antibiotic resistance, they agree that the disinfectants have been necessary, especially during the pandemic. “We needed something very quickly when this pandemic erupted,” McNamara says. “We didn’t necessarily have time to understand the ramifications of each individual chemical that could have been used for a disinfectant; it was just important to have disinfectants.”
Because we will continue to need disinfectants, some researchers are looking for treatment processes that can efficiently break down quats in wastewater treatment plants. “We should find ways to remove them so bacteria don’t get exposed, in order to prevent or limit antibiotic resistance,” Konstantinidis says.
At Arizona State University, Yenjung Sean Lai had been working on a reactor that uses a biofilm of microbes growing on a membrane to biodegrade organic contaminants. When he and colleagues tested the reactor on the quat hexadecyltrimethylammonium bromide, they found that the system could degrade almost all the compound in influent water, even when the concentrations were high enough for disinfection (Water Res. 2017, DOI: 10.1016/j.watres.2017.07.003). But there was an unforeseen side effect—antibiotic-resistance genes proliferated in the biofilm, Lai says.
Advertisement
In a follow-up study, Lai and his colleagues tested different operating conditions and found that the rise in antibiotic-resistance genes was hard to avoid (Sci. Total Environ. 2021, DOI: 10.1016/j.scitotenv.2020.144264). Lai suggests that oxidizing quats prior to biological treatment could make them harmless to bacteria and avoid the development of resistance, particularly in plants that receive quat-rich wastewater from health-care facilities or slaughterhouses.
At Boğaziçi University, Tezel’s approach is to use quat-resistant Pseudomonas bacteria to consume quats (J. Hazard. Mater. 2021, DOI: 10.1016/j.jhazmat.2021.126210). The team had previously identified a highly quat-resistant species, BIOMIG1, and trapped the bacteria inside alginate beads. The researchers then built a bioreactor that uses a column filled with the beads to break down quats as wastewater flows through.
Tezel is now working to determine if quat-degrading genes from these microbes can be transferred between bacteria. While rapid biodegradation of quats is desirable in wastewater treatment plants, this ability could be problematic if the trait reaches bacteria in the wider environment. That could render quats ineffective where they are needed, such as in hospitals.
These researchers hope that their efforts could help avoid unintended long-term consequences of quat use, such as the spread of antibiotic resistance, and help quats remain effective disinfectants for years to come.
XiaoZhi Lim is a freelance writer based in Massachusetts.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter