Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
The world needs water. These materials take it from the air
Hydrogels and other polymers join metal-organic frameworks as options as researchers expand the possible applications of harvesting water from the atmosphere
by XiaoZhi Lim, special to C&EN
April 25, 2021
| A version of this story appeared in
Volume 99, Issue 15
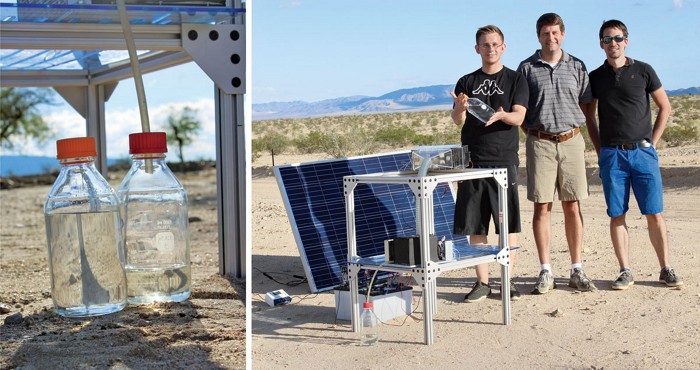
Every evening during the summer of 2020, Xingyi Zhou went up to the roof of her lab building at the University of Texas at Austin to check on two plastic boxes containing radish plants.
Plants in one of the boxes languished, while those in the other flourished, even though both grew in sand that Zhou and her colleague Fei Zhao collected from an Austin park. The key difference was a ground-up slab of a hydrogel mixed in the sand of one of the boxes. The tiny grains of hydrogel absorbed moisture from the cool, humid night air. In the mornings, the hydrogel gradually warmed up under the heat of the sun and released the water it had absorbed overnight, generating a humid, healthy environment for the plants (ACS Mater. Lett. 2020, DOI: 10.1021/acsmaterialslett.0c00439).
Materials like this UT Austin hydrogel could someday help mitigate the world’s water woes. Four billion people around the world face water scarcity at least 1 month a year, according to a 2016 study (Sci. Adv., DOI: 10.1126/sciadv.1500323), and a hotter, drier world brought about by climate change is expected to exacerbate the problem. The potential for harvesting water from the air to address this scarcity is immense. Some studies suggest that the amount of water contained in the atmosphere is roughly equivalent to 10% of the fresh water held in lakes.
Technologies already exist to catch fog or collect dew that condenses overnight. But those techniques are typically limited to regions where fog forms and to climates with large temperature swings between day and night. In recent years, materials that can absorb the water held in the air are proving to be more versatile and deployable in diverse ways. These advances in water-absorbing materials partly spurred the US Defense Advanced Research Projects Agency (DARPA) to commit $45 million to six projects in the Atmospheric Water Extraction program in November 2020, program leader Seth Cohen says. DARPA is looking for portable, low-energy devices that produce drinking water from the atmosphere under a range of climate conditions to support troops and, in the longer term, civilians and humanitarian missions, Cohen says.
Materials will play a key role in any successful water-from-air device. In 2017, one material, metal-organic frameworks (MOFs), made a splash in this research field. These porous solids consisting of metal ions linked by organic molecules can capture atmospheric moisture in very dry air and release that water with moderate solar heating. Omar Yaghi, a materials scientist at the University of California, Berkeley, says any serious contender for atmospheric water harvesting needs to perform well in arid regions. “That’s where people who need it are,” says Yaghi, who is one of DARPA’s grant recipients.
But other researchers, including Zhou and Zhao’s group led by Guihua Yu at UT Austin, have taken another approach. The Yu group, another recipient of DARPA funding, has begun using polymers to create hydrogels that it hopes could rival MOFs. Hydrogels don’t have the water-grabbing abilities of MOFs in arid conditions and have not been tested for long-term stability, but they can hold more water in humid conditions and can be tuned by pairing with other materials, like desiccants and even MOFs. And beyond harvesting water from air for drinking, researchers want to use water-absorbing materials in other applications, such as self-irrigating soil and air-conditioning. “It’s almost endless applications,” Yaghi says.

Newfound flexibility
When Zhou and Zhao joined Yu’s group at UT Austin in 2016, the lab wasn’t focused on harvesting water from the air. Instead, the researchers worked on purifying brackish water or wastewater by heating it under the sun to generate vapor, then condensing the vapor into clean water.
But Zhou realized that many resource-limited regions may not have access to any type of water. So she suggested to the group that generating water from the atmosphere could be more widely applicable.
The team set to work. Zhao started with a polypyrrole that the researchers had used to absorb solar heat for generating water vapor in wastewater purification. She then doped it with chlorine to turn it into a hygroscopic polymeric salt. The researchers combined that polymer with poly(N-isopropylacrylamide) (PNIPAM), a thermal-responsive polymer, which they had used before to create materials for controlled drug release. At room temperature, PNIPAM is hydrophilic and stays straight and linear. But once above a critical temperature, around 37–40 °C, PNIPAM coils up and becomes hydrophobic. This thermal switching ability is key, Yu says. Hydrophilic PNIPAM helps store the water absorbed by the hygroscopic polymeric salt; hydrophobic PNIPAM repels and squeezes out the collected water.
This combination of the hygroscopic polymer and PNIPAM resulted in what the Yu group calls a super moisture-absorbent gel (SMAG) (Adv. Mater. 2019, DOI: 10.1002/adma.201806446). At 90% relative humidity, 1 g of dry SMAG can hold 6.7 g of water, becoming saturated in approximately 100 min. Over 28 cycles, each lasting 1 h, 1 g of SMAG cumulatively produced 55 g of water in an environment with 75% relative humidity.
The SMAG’s performance plummets in dry conditions, however. At 30% relative humidity, 1 g of the hydrogel needs 180 min to take in just 0.7 g of water. In comparison, 1 g of an aluminum-based MOF called MOF-303 needs just 3 to 10 min to bring in 0.4 g of water at 20–40% relative humidity.
Yu admits that MOFs perform better than the SMAG at low humidity. But he thinks that at high humidity, hydrogels have an edge because they can expand to hold more water. For instance, an application like self-irrigating soil needs a material that can continually take in water overnight, store it, and slowly release water to irrigate crops during the day.
MOFs can’t expand to fit large amounts of water, says Peng Wang, a materials scientist at King Abdullah University of Science and Technology (KAUST). MOFs have a fixed pore volume for containing water, whereas hydrogels are flexible. Hydrogels do not “limit how much water vapor the whole system can take.”
Yaghi disagrees that MOFs’ fixed porosity is a disadvantage, particularly when it comes to producing drinking water. “The hydrogels have very large pore volume, but if you can’t extract water from low humidity, then what’s the use of that pore volume?” He notes that if a material absorbs well at low humidity, it will perform even better at high humidity, and researchers can compensate for a lack of pore space by cycling through the water absorption and release processes, provided those processes are fast.
Some researchers are looking to improve hydrogels’ performance at low humidity. One solution involves hybridizing hydrogels with desiccant salts. Traditional hygroscopic desiccants like silica gel, calcium chloride, and lithium chloride are cheap, giving them an advantage in harvesting water from the atmosphere at large scale, says Ruzhu Wang, a mechanical engineer at Shanghai Jiao Tong University. But these desiccants are difficult to use because they turn into liquid as they take in water. Some research groups have begun incorporating them into hybrid materials, using polymers to hold the salts in a gel form.
One of the first reports of hybrids came in 2018 from a German team, which used cheap alginate polymers derived from algae to bind calcium chloride into solid hydrogel beads (Commun. Chem. 2018, DOI: 10.1038/s42004-018-0028-9). These beads can absorb their own weight in water at 26% relative humidity but require heating to at least 100 °C to release the water.
Around the same time, KAUST’s Wang prepared a composite hydrogel of polyacrylamide with calcium chloride and carbon nanotubes (Environ. Sci. Technol. 2018, DOI: 10.1021/acs.est.8b02852). The calcium chloride salt absorbs the water, while the carbon nanotubes help absorb solar heat, assisting with water release. Under controlled experiments, at 35% relative humidity, 1 g of the composite absorbed 0.74 g of water and released almost all of it under the hot sun.
Plenty of places with high humidity are also water stressed. Actively condensing that muggy air with a dehumidifier is already viable in these regions, but that approach requires significant amounts of energy. So Yaghi thinks that water-harvesting materials that are limited to working at high humidity need to operate completely without energy input to be practical. “You need a material that works by thermal cycling without plugging it into the wall.”
Cool aid
Other than harvesting water for drinking, materials that passively absorb water in the air could also help in some cooling applications.
In hot, humid Singapore, Ghim Wei Ho, a nanomaterials scientist at the National University of Singapore, has been collaborating with a cooling company to develop a material that could dehumidify moist air before it passes through an air-conditioning system. Almost half of buildings’ energy consumption in Singapore is devoted to air-conditioning. Passively dehumidifying the air before cooling it could help save energy by lowering the air’s dew point, thereby increasing air conditioners’ efficiency.

When Gamze Yilmaz was a postdoctoral researcher in Ho’s lab, she worked on a hybrid material combining a chromium-containing MOF called MIL-101, calcium chloride, and PNIPAM. One morning, she found her samples in a puddle of water. She had stumbled on a formula that captured and released water simultaneously (Sci. Adv. 2020, DOI: 10.1126/sciadv.abc8605). When the gel is left alone on a table, beads of water appear on the gel without any temperature changes to trigger water release, Ho says. The material continuously releases about 80% of its absorbed water without heating. If the material is warmed to 32 °C, the remaining 20% gets expelled.
To test the material further, the researchers mounted 12 flat disks of the MOF-polymer-salt hybrid onto a metal plate held at an angle, allowing the released liquid water to run down a tube into a cylinder. Outdoors, 1 g of the hybrid material produced 4.2 g of water in a day.
Ho envisions integrating the hybrid material within a commercial air duct system. Humid air drawn into the system could first pass through this material, which would absorb water; then the air could move on for cooling. The passive dehumidification would save energy on air-conditioning, Ho says, and the collected water could be used to meet the building’s needs.
KAUST’s Wang and his colleagues attempted a different cooling application with their water-harvesting hydrogel: directly cooling solar panels (Nat. Sustain. 2020, DOI: 10.1038/s41893-020-0535-4). Photovoltaic panels heat up under the sun, particularly in urban or desert settings. This heating is detrimental—with every 1 °C rise in temperature, panels lose 0.5% in sun-to-electricity efficiency. Wang’s team attached its hybrid of polyacrylamide and calcium chloride to the back of a small photovoltaic panel. At KAUST’s campus on two August days in 2019, the material kept the panel about 10 °C cooler than one without it and increased the panel’s efficiency by 19%.
Future directions
DARPA hasn’t set specific performance goals for water-from-air harvesting devices, Cohen says. But the agency plans to test these devices under a range of climate conditions, such as at 27 °C and 10% relative humidity, 43 °C and 60% relative humidity, and 4 °C and 50% relative humidity.

For now, hydrogels and hybrids are still far behind MOFs when it comes to low-humidity conditions. Back in October 2018, Yaghi’s team brought a working device based on MOF-303 to the Mojave Desert, where daytime relative humidity drops to 10%. The device operated continuously for 3 days, producing an average of 700 g of water per kilogram of MOF-303 each day (ACS Cent. Sci. 2019, DOI: 10.1021/acscentsci.9b00745). With a spin-off company Water Harvesting, the team has since integrated 100 g of MOF-303 into a small device that delivers 4 L of water per day—enough for a person to survive on. The device has been running continuously for about 30,000 water-absorbing-and-releasing cycles without noticeable drops in performance, and the team expects the device to last 5–6 years without needing to replace the MOF.
Yaghi thinks that to be a practical solution, any water-harvesting material will need to demonstrate long-term stability over many cycles of water absorption and release. His team has also begun exploring a class of metal-free, crystalline materials called covalent organic frameworks, or COFs, that could last longer than MOFs (J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.9b13094).
KAUST’s Wang admits that so far, he does not have data to determine the long-term stability of his team’s hydrogel. Meanwhile, Yu says that his team has grown plants in the same SMAG-containing soil for over a month and plans to conduct longer-term experiments.
Advertisement
While MOFs have gotten good at absorbing water, the last step in the harvesting process could use improvement, says Qiaoqiang Gan of the University at Buffalo. Absorbent materials like MOFs can only take in and store water, Gan says. After that, the material needs to be heated to drive the water out, and the hot vapor then needs to be condensed to collect the liquid.
Zongfu Yu at the University of Wisconsin–Madison and Gan, who together launched a solar water purification company in 2016, believe that this condensation step will be the performance-limiting factor when it comes to scaling up water production technologies, like solar water purification and water-from-air harvesting. When these techniques release large quantities of water vapor, active cooling is often needed to quickly condense the vapor to keep up with the rate at which it’s generated. But active cooling requires energy, which makes the system less efficient overall.
To condense water without using energy, Gan’s and Yu’s teams developed a passive radiative cooling material by layering thin films of polydimethylsiloxane and aluminum (Proc. Natl. Acad. Sci. U.S.A. 2021, DOI: 10.1073/pnas.2019292118). This material can condense water throughout the day without energy input.
Condensation is less of a problem for some hydrogels, like the SMAGs, because they release water in liquid form, not as vapor.
To help hydrogels become more practical and catch up to MOFs’ water-harvesting abilities at low humidities, Guihua Yu’s group at UT Austin is exploring entirely new polymers to create second-generation SMAGs. One bigger issue that DARPA isn’t asking about but that concerns Yu is cost. Polypyrrole and PNIPAM are both relatively expensive, he says, so they are less suitable for agricultural applications, which require cheap materials.
For now, Zhou continues to test the first-generation SMAG-soil mixtures on the lab-building rooftop. She recently harvested microgreens from the boxes and added them to her salad; soon she will harvest pea plants. She hopes to further improve the SMAG-soil combination to deliver not only water but also nutrients for plants. The team aims to create a material that could eventually help combat desertification around the world. “Many lands become really inhospitable for plants to grow because of climate change,” Yu says. Maybe before long, both humans and crops can drink from the atmosphere.
XiaoZhi Lim is a freelance writer based in Massachusetts.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter