Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
2-D Materials
Huge 2-D metallo-supramolecule
Self-assembled supramolecular grid measures 20 nm across
by Laura Howes
April 19, 2020
| A version of this story appeared in
Volume 98, Issue 15
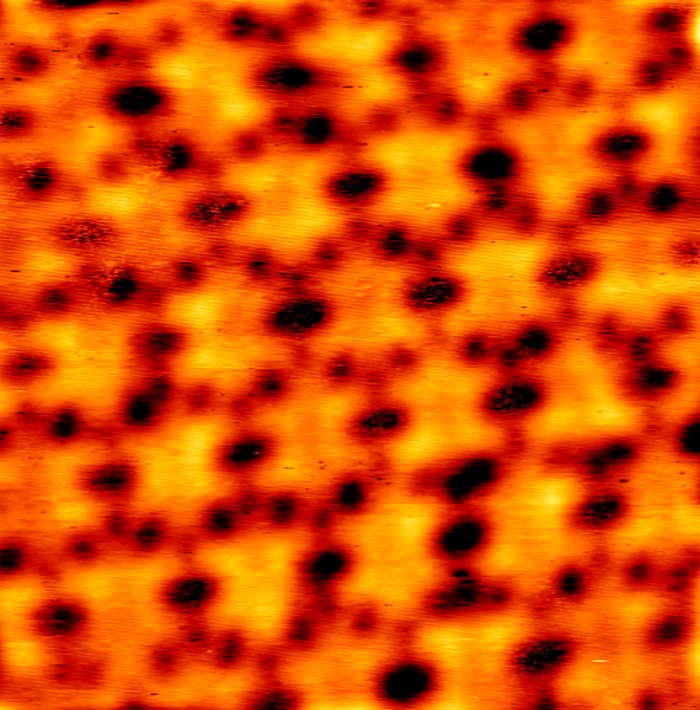
In its quest to make a really big 2-D nanostructure, Xiaopeng Li’s University of South Florida team pulled out all the stops. It used both intra- and intermolecular self-assembly to build a grid of hexagons held together with ruthenium and iron ions. This monster of a metallo-supramolecule, made with help from collaborators at Argonne National Laboratory, Ohio University, and many other institutes, measures about 20 nm across (Nat. Chem. 2020, DOI: 10.1038/s41557-020-0454-z). Li says it ranks among the largest discrete metallo-supramolecules produced to date. Each hexagon of the grid is made from organic linkers that stick out in three directions. At all three ends of the spiky molecule are the nitrogen-containing terpydine ligands that complex ruthenium ions. These combine until you get a hexagonal building block with some unbound ligands. Add iron(II), and the hexagons start snapping together to make a linked-up lattice. The iron ions’ positions can produce different isomers. This giant molecule is more than a novelty. The team hopes to replace the iron with other metal ions, such as cobalt, to produce variations that can act as single-molecule information storage devices by using different spin states.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter