Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
2-D Materials
Introducing borophane, a chemically stable form of borophene
Hydrogen treatment prevents oxidation, making material easier to process for applications
by Mitch Jacoby
March 26, 2021
| A version of this story appeared in
Volume 99, Issue 11

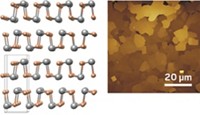
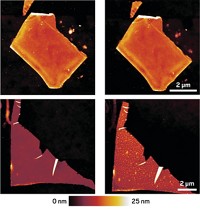
By modifying borophene with hydrogen, researchers have converted the atomically thin, unstable 2D form of boron to a chemically stable form dubbed borophane. The work makes the ultrathin form of boron more robust and easier to handle, opening the door to applications (Science 2021, DOI: 10.1126/science.abg1874). As various types of 2D materials started to stack up about 10 years ago, researchers wondered whether they could wrangle boron, an inherently 3D material, into a 2D form. Theoretical studies suggested that borophene would be mechanically strong and flexible and have electronic properties that could be useful in fast electronics and energy storage devices. In 2015, two teams succeeded in preparing borophene samples by growing 2D films in chemically controlled environments. The problem, according to Northwestern University’s Mark C. Hersam, a member of one of those teams, is that borophene oxidizes immediately upon exposure to air, making it nonconductive and ruining other potentially useful properties. Hersam and coworkers now report that treating borophene with atomic hydrogen in vacuum renders the material stable in air for about 1 week. The team found that the hydrogenation step is reversible, suggesting it could be used to protect the material during ambient processing, then undone to restore pristine borophene after the film has been encased in an electronic device.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter