Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
2-D Materials
New method deposits low-resistance electrodes on 2-D semiconductors
Van der Waals contacts between materials could lead to high-performance transistors
by Prachi Patel, special to C&EN
March 28, 2019
| A version of this story appeared in
Volume 97, Issue 13

As silicon transistors get harder to shrink, researchers are turning to 2-D semiconductors to try to continue producing tiny, powerful electronics. But attaching metal electrodes to atoms-thick 2-D materials is a challenge. The two materials don’t mesh well, and the high resistance at their interface drags down the device’s speed and power. Researchers have now made a smooth, low-resistance metal electrode on a single molecular layer of molybdenum disulfide, allowing them to build high-performance transistors with the 2-D material (Nature, 2019. DOI: 10.1038/s41586-019-1052-3).Researchers typically deposit gold or titanium electrodes on 2-D semiconductors in an electron-beam evaporator, a machine that uses an intense electron beam to vaporize a metal inside a vacuum. In the process, water or other contaminants get trapped at the interface between the electrodes and semiconductors, creating defects that slow down electrons. The metals can also damage the semiconductor. Gold atoms, which have a lot of kinetic energy when the metal vaporizes, can break the 2-D layer, while titanium can react with it.
For a defect-free contact, scientists have transferred metal films or graphene-flake electrodes onto 2-D semiconductors. The different materials stick to each other with van der Waals forces. But the method has limits, says Yan Wang, a graduate researcher in materials science at the University of Cambridge. “You can only do small regions, and it can take half a day to make the dry-transfer contact on one device.” Plus these strategies have only been used for multi-layer 2-D semiconductors.
Wang, materials scientist Manish Chhowalla, and their colleagues report a way to quickly create metal electrodes on a single layer of molybdenum disulfide using a standard laboratory electron-beam evaporator. The researchers first grow single-layer molybdenum disulfide on a silicon substrate. They place the sample in the evaporator and coat it with a 10-nm layer of indium followed by a 100-nm gold layer. The two metals form an alloy that makes van der Waals contact with the semiconductor.
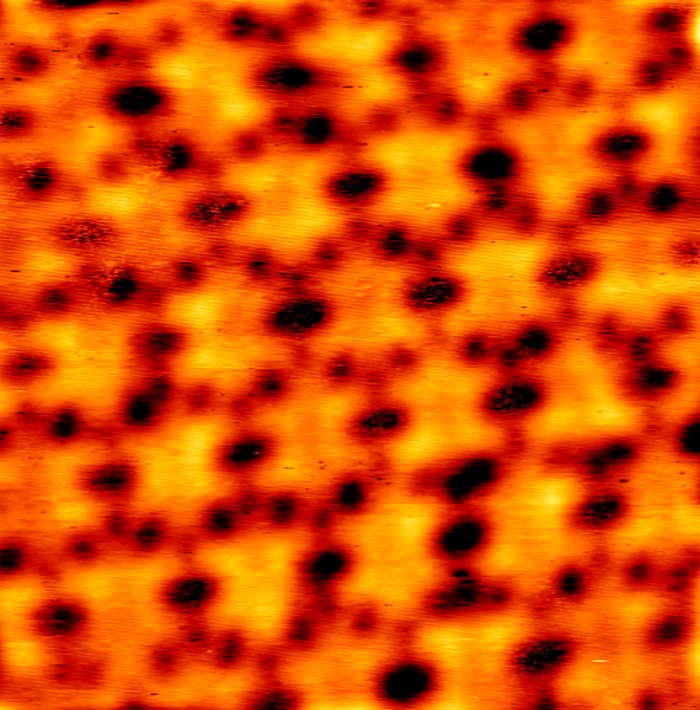
Atomic-resolution microscopy imaging showed that the method deposited a clean indium layer on top of undamaged molybdenum disulfide. The indium acts like a protective barrier, shielding the semiconductor from the kinetic energy of gold atoms. The shiny silver metal is inert and does not react with the semiconductor. And it has a relatively low melting point of about 157 °C, allowing the researchers to use a low-energy electron beam to vaporize it. “That means there is less kinetic energy in the indium metal so it deposits gently on the sample without causing damage,” Wang explains.
The resistance at the interface is some of the lowest for metal electrodes evaporated onto 2-D molybdenum disulfide, Wang says. This leads to transistors with high charge-carrier mobilities, which are needed for high-performance devices. The team also deposited the metal electrodes on few-atomic-layer films of other 2-D semiconductors such as tungsten disulfide and tungsten selenide.
Contact resistance is one of the biggest hurdles to making high-performance transistors, and the electronics industry has worked for more than three decades to minimize it in silicon devices, says Deep Jariwala, a materials scientist and engineer at the University of Pennsylvania. “We are only getting started with 2-D semiconductors,” he says.
Advertisement
“This is a very exciting advancement that might be compatible with conventional electronic fabrication processes,” says Xiangfeng Duan, a chemist at the University of California, Los Angeles. However, he says, it would be important to expand the approach to other metals with slightly higher melting points than indium. The metal’s low melting temperature could pose a challenge for devices that heat up under high-current, high-speed operation.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter