Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chemists debate how to fuel molecular machines
As researchers develop efficient molecular fuels, they continue to wrestle with the fundamentals of how their machines work
by Mark Peplow, special to C&EN
February 5, 2023
| A version of this story appeared in
Volume 101, Issue 5
In brief
Artificial molecular machines now come in a dizzying variety of forms, including motors, pumps, and data-reading ratchets. While some machines are powered by light, a growing number are driven by chemical reactions with fuel molecules. Chemists are now learning how to fine-tune these chemical fuels to improve the machines’ performance and are building autonomous machines that keep ticking as long as they have a fuel supply. But the work is also fueling debate about the machines’ fundamental operating principles. As researchers grapple with the kinetics and thermodynamics of molecular machines, many believe that developing a more robust set of design principles for these nanosized devices will ultimately help the field.
The workshops of molecular machinists seem to be growing louder by the year. You can almost hear the imaginary whirring of their nanoscale devices, accompanied by an anvil chorus of tiny but industrious hammers.
Over the past couple of decades, these researchers have assembled a dazzling array of molecules with moving parts that act like miniature machinery. They’ve made motors with spinning paddles, pumps that gather molecules from solution, molecular assemblers that put together peptides, and ratchets that can read data stored on strands of molecular tape. Pioneers of this work even garnered a Nobel Prize in Chemistry, awarded in 2016.
For much of that time, one of the field’s overriding questions has been, What can these devices do? And although applications are still relatively distant, researchers are beginning to see how molecular machines might be harnessed for useful tasks. Molecular motors can flex nanofibers or rearrange liquid crystals, for example, which can be used to create responsive, smart materials.
Now researchers are turning to deeper questions: How do the machines work, and how can we improve them? The answers, some say, will come from probing these systems’ underlying kinetics and thermodynamics to understand how energy and competing reaction rates make them tick.
Grappling with these fundamentals could help the field move beyond a somewhat trial-and-error approach to machine construction and instead develop a more robust set of design principles. “It happens quite regularly in this community: people have a great idea, they synthesize it for 2 years, and then it doesn’t work,” Elisabeth Kreidt, an inorganic chemist at TU Dortmund University, says. “That’s a huge limit to the field because it consumes so much time and resources.”
Researchers are already learning how to fine-tune the chemical fuels that drive some molecular machines. They are also building autonomous molecular machines that require far less intervention from their human operators, chugging along happily as long as a carefully chosen fuel supply is present. “The more we find out about the theory, the better we can design fuels,” David Leigh of the University of Manchester says.
But this focus on fuels is also stirring heated debate about the fundamental principles of how these machines run. “Some people in the community literally think these chemicals are putting energy into their machines, which some of us really disagree with,” Ivan Aprahamian of Dartmouth College says. “We even disagree about what a machine is.”
These may sound like abstract arguments, but they go right to the heart of whether molecular machines can be truly useful. “It’s very important because if you don’t get the thermodynamics right, your molecule will move but you will never be able to extract work from it,” Nathalie Katsonis, a chemist at the University of Groningen, says.
Power up
Motors are perhaps the most iconic type of molecular machine. They typically operate by stepping through a repeating cycle of chemical reactions that change the shape of the molecule and cause movement—rotation around a bond or motion along a track, for example. To prevent the motor from twitching uselessly back and forth, researchers also need a mechanism to ensure it moves in only one direction.
The first fully functioning, synthetic rotary molecular motor was unveiled in 1999 by Ben Feringa of the University of Groningen. Feringa shared the 2016 chemistry Nobel Prize with Jean-Pierre Sauvage of the University of Strasbourg and Sir Fraser Stoddart of Northwestern University for their work on molecular machines.
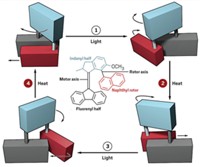
Feringa’s groundbreaking motor contained two bulky chemical groups connected by a carbon-carbon double bond. The groups rotated around this axle through a series of isomerizations induced by ultraviolet light and heat. The motor molecule’s chirality ensured that the bulky groups could squeeze past each other when they moved forward in the cycle but not backward (Nature 1999, DOI: 10.1038/43646).
Dozens more light-driven motors followed in its wake, and they’ve been harnessed for a variety of tasks, such as producing light-responsive gels and moving artificial muscles. Light is a convenient and tunable energy source, Feringa says, and it doesn’t produce waste products.

Yet for those seeking to understand and mimic biological molecular machines, such as motor proteins that help transport cargo inside cells, light falls short. Biology has successfully used molecular pumps and motors for billions of years but generally drives them with chemicals such as adenosine triphosphate (ATP) rather than light. For chemists, that precedent poses an irresistible challenge to develop synthetic molecular machines that are fueled by chemical processes, Feringa says.
In 1999, T. Ross Kelly of Boston College took an important step toward that goal by developing a phosgene-fueled prototype motor that could turn 120° (Nature 1999, DOI: 10.1038/43639). Six years later, Feringa built a chemically driven rotary motor that could complete a full revolution around a single C–C bond by forming and breaking a lactone that bridged the motor’s two units. A chiral reducing agent served as the fuel, opening the lactone and ensuring that the motor rotated in a single direction (Science 2005, DOI: 10.1126/science.1117090).
Researchers now have a range of other fueling strategies (ChemistryOpen 2022, DOI: 10.1002/open.202200128). Some employ a series of protection and deprotection steps that add or remove hefty chemical groups to the machine. Others vary the pH to move a machine through a complete cycle. A third approach, developed by Stoddart in the 1990s, depends on oxidation and reduction reactions.
Most of these chemically driven devices rely on their human handlers to add the right kind of fuel or other reagents at each point in the machine’s cycle. Stepwise operation may be fine for some applications, but it’s also labor intensive—like hand cranking an old jalopy.
More importantly, it is not how biomolecular machines work. They swim in a sea of fuel molecules such as ATP, pick them up whenever they need one, and operate continuously. Achieving that kind of autonomy in synthetic molecular machines is an important goal for the field, Stoddart says. “We need to have the autonomous operation that we see in our own biological motors.”
Unlike photons, chemical fuels provide a way to store and transport a concentrated energy source that molecular machines can access on demand (Nat. Chem. 2022, DOI: 10.1038/s41557-022-00970-9). Leigh believes that if the machines can access an energy source as needed, they could have a wider range of applications than nonautonomous systems. “If there is going to be a nanorevolution based on molecular machines, it’s likely to be powered using chemical fuels,” he says.
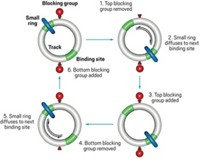
In 2016, Leigh unveiled a key milestone in the push for autonomy. “It was the first autonomous chemically driven motor,” he says. The motor consists of a molecular ring that can move around a circular track. There are two regions, called recognition sites, on opposite sides of the track, which can hold the ring in place by hydrogen bonding. Each recognition site sits next to a hydroxy group that reacts with a fuel, fluorenylmethoxycarbonyl chloride (Fmoc-Cl). This reaction installs bulky Fmoc groups on the track, blocking the ring’s movement (Nature 2016, DOI: 10.1038/nature18013).
But the reaction mixture also contains a base that helps rip off the blocking groups, allowing the ring to pass. The upshot is that the Fmoc groups constantly pop on and off the track’s hydroxy anchor sites. Crucially, steric hindrance ensures that the reaction to add an Fmoc happens about five times as fast at the anchor site that is opposite the ring. This asymmetry in reaction rates, sometimes called kinetic gating, ensures that the ring is more likely to move clockwise rather than counterclockwise around the track.
As molecular motors go, it’s not especially fast or efficient. “This is absolutely a poor rotary motor,” Leigh says. “But it’s one of the most important papers that we’ve published because it shows how molecular motors work, and it shows how to design molecular motors that can function autonomously.”
Since then, Leigh has used similar Fmoc fuel chemistry in an autonomous pump that gathers crown ethers from solution and puts them onto a long storage chain (Nature 2021, DOI: 10.1038/s41586-021-03575-3). The advent of such autonomous devices has caused excitement, but it also feeds into a long-running debate about how chemically driven molecular machines actually work. And that’s where things get tricky.
Fuel fuss
To understand that debate, consider kinesin, a protein machine that carries cargo within cells. The protein has two “feet” that stride along stiff tracks called microtubules, and the motion is driven by the hydrolysis of ATP into adenosine diphosphate (ADP). Some researchers have argued that breaking the strong phosphate bond in ATP primes kinesin by putting it into a high-energy state. Kinesin’s relaxation from this state—a process described as a “power stroke”—causes a conformational change that kicks the feet forward, one after the other.
“But that’s wrong. And I say this as a deductive fact, not as an opinion,” says Dean Astumian, a physicist at the University of Maine who has played a key role in guiding the thinking about the operation of molecular motors.
Instead, he says, experimental data show that kinesin’s motion is controlled by the relative rates of reversible reactions involving kinesin, ATP, and their products. Each foot’s movement is impelled by the Brownian motion of surrounding molecules rather than a direct jolt of energy. The reactions between ATP and kinesin determine which foot rises up from the track, and they selectively block backward motion. Chemists call this kind of system a Brownian ratchet. (ChemPhysChem 2016, DOI: 10.1002/cphc.201600184).

“There’s a fundamental difference between how molecular machines and macroscopic machines work,” says Kreidt, who previously worked in Leigh’s lab. In a macroscopic motor, you use the energy in a fuel to overcome inertia. But in a molecular motor, everything is already moving thanks to Brownian motion—so you need the fuel’s energy to put the motor in a state in which the motor can move in a particular direction.
Astumian and others argue that all chemically driven molecular machines are governed by the kinetic asymmetry in this kind of Brownian ratchet. In contrast, power strokes are involved in driving light-driven machines—light excites the device into a high-energy state, and its relaxation causes a large mechanical change.
This distinction in mechanism has major implications. “The design principles for light-driven motors are entirely different than for catalysis-driven motors. If you fail to appreciate that, you’re going to waste an awful lot of experimental time trying to do things that are fundamentally impossible,” Astumian says.

The Brownian ratchet model is widely accepted among the molecular machinists. But Aprahamian points out that if a fuel molecule’s primary role is not to supply energy to make a molecular motor move, it should not be called a fuel at all. In a recent preprint article that has not been peer-reviewed, he and Stephen Goldup of the University of Southampton argue that researchers must abandon talk of fuels altogether because the term is mechanistically misleading (ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-49s4d). To promote the paper, Aprahamian tweeted a version of a popular internet meme that neatly sums up his position: a photograph of someone sitting at a desk with a poster that reads, “Your chemical is not a fuel! CHANGE MY MIND.”
Some machinists aver that fuel is merely a convenient shorthand for any reagent that drives a molecular machine. Others have tried to find common ground by developing more nuanced definitions—which may not have helped. “There have been at least 10 different reviews about this recently, and each one of them has a completely different definition of a chemical fuel,” Aprahamian laments. “It’s basically a Tower of Babel.”
Advertisement
To wrestle with this and other mechanistic issues, researchers have recently outlined a series of models that describe the underlying thermodynamics and kinetics of molecular machines. Kreidt points out that these model-based approaches can help calculate key parameters of a motor, such as its directionality, before it is built. Estimating the amount of kinetic asymmetry in the system should predict how consistently it will turn in one direction, for example. “So we don’t always need to prepare the full molecular motor, which can be extremely time consuming, without having a clue if the system will actually work,” she says.
Leigh thinks that a proliferation of models that help chemists think about molecular machines is useful. “They will all be true descriptions of how that machine works,” which could help to settle debates about fuels and power strokes, Leigh says. But Astumian is less convinced. “Some of them are just wrong,” he says.
So the debate about these models’ merits rumbles on. But Leigh says that they are already helping shape the designs of new molecular machines.
Designer fuels
In April, Leigh unveiled a chemically driven autonomous motor that is much more efficient than his 2016 example. The motor benefits from an improved fueling reaction and two distinct steps that each confer some kinetic asymmetry to its reaction cycle.
The motor contains a pair of aryl groups that rotate around a C–C single bond by forming and breaking a bridging anhydride group. The researchers use a chiral carbodiimide fuel and a chiral hydrolysis catalyst to create and destroy the anhydride, ensuring the motor (mostly) turns in one direction (Nature 2022, DOI: 10.1038/s41586-022-04450-5).
Meanwhile, Feringa says his team is using molecular mechanics calculations and other modeling approaches to guide machine designs and to understand how different structures and substituents could make them work faster and more efficiently. In July, the researchers showed off an autonomous motor that runs on carbodiimide and acid but rotates by forming and breaking a bridging ester (Nature 2022, DOI: 10.1038/s41586-022-05033-0). In this case, the motor molecule’s chirality controls the machine’s directionality.


Leigh is also modifying his fuels to enhance their performance. In September, his team demonstrated that approach on an autonomous ratchet that contains a ring trapped on a linear track (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c07633). The track has two recognition sites that can grab the ring and an anchor point for a benzotriazole blocking group next to one of the recognition sites. A carbodiimide fuel puts the blocker in place, and the reaction conditions remove the blockers by hydrolysis.
This setup also boasts two ways of controlling the machine’s kinetic asymmetry. First, the bulky blocker is more likely to bind to the track when the ring is at the more distant recognition site, because of steric hindrance with the carbodiimide fuel. Second, the blocker is more likely to pop off when the ring is at the neighboring recognition site, thanks to hydrogen-bonding activation. The result is that after a few hours, the ring is about 20 times as likely to end up at the recognition site farther from the barrier.
Leigh’s team found that using a bulkier carbodiimide fuel raised the distribution ratio of where the ring ends up to 30:1 and that adding an ammonium group to the end of the fuel molecule made the machine work faster. In principle, similar modifications could be used to give any chemically fueled machine a boost, Leigh says.
For now, all these chemically fueled autonomous machines are proof-of-principle devices that do not perform useful tasks. But Leigh is working on it. In collaboration with the University of Groningen’s Katsonis, for example, he hopes to manipulate liquid crystals with his chemically fueled motors, something already achieved with light-driven motors. Using the motors to reorient arrays of crystals can change their optical properties, for example, which might form the basis of adaptive materials or optoelectronic devices.
Katsonis says that project is highlighting one of the difficulties for chemically fueled systems: how to deal with their waste products. The chemical waste produced by fueling reactions can alter reaction conditions such as pH or bind to the machine in ways that hamper access to further fuel molecules. “If you cannot remove the waste material, then the efficiency of the whole system will decrease with time,” says Soumen De at the Indian Institute of Science Education and Research Thiruvananthapuram. “That is a major problem, and future research will definitely focus on how to decrease the waste or how to reuse it.”
Waste molecules might be recycled back into fuel, for example, just as nature turns ADP back into ATP. To achieve that, researchers will need to develop a broader palette of fuels for autonomous machines. “We definitely have to find more fueling reactions so that our toolbox is enriched,” De says.
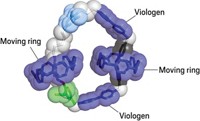
Meanwhile, another power source is coming to the fore. Stoddart recently developed a molecular motor that uses a redox mechanism driven directly by electricity (Nature 2023, DOI: 10.1038/s41586-022-05421-6). “We prefer to use electricity because that comes with no waste products,” Stoddart says.
The motor contains two rings that move around a circular track in response to an oscillating voltage, and the system can complete a full circuit in just a few minutes. His team is now trying to fix one of the rings to a surface so that electricity makes the inner track roll around. “If we can attach millions of motors to a surface, it could move a solution across the surface,” says Stoddart’s Northwestern colleague Long Zhang, who led experimental work on the motor.
Faced with healthy competition from light and electricity, chemically fueled autonomous machines now need to show that they can perform a variety of useful mechanical functions, Feringa says. “That’s the most interesting, important thing—and that’s the big challenge.”
Mark Peplow is a freelance science journalist based in Penrith, UK.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter