Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Tugging on this molecule changes its color
Fast-acting mechanophore could be used to sense when a polymer is at its breaking point
by Bethany Halford
March 11, 2021
| A version of this story appeared in
Volume 99, Issue 9
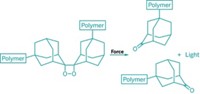
Polymers could one day signal they’re about to break by changing color, thanks to a new, fast-acting mechanophore based on oxazine. Mechanophores are molecules that can be inserted into polymers and will make or break bonds in response to physical forces on the polymer. Although they’ve been around for more than a decade, most mechanophores are relatively slow to react, and once they change, they don’t necessarily go back their original state—properties that have limited their use.
This was the case with a spiropyran mechanophore first reported by Jeffrey S. Moore’s and Nancy R. Sottos’s labs at the University of Illinois at Urbana-Champaign. While the spiropyran would change a polymer’s color when stretched, it didn’t do so quickly and the change wasn’t entirely reversible. “It missed out on the ability to really be a molecular probe to sense what’s going on when it’s going on,” Moore says.
Hai Qian read that paper as a graduate student and wrote to Moore proposing to explore oxazines as mechanophores in a postdoctoral fellowship. Oxazines, which can toggle between states in response to triggers like pH, have a much simpler, faster switching mechanism than spiropyrans, which rely on complex ring-opening and ring-closing maneuvers that involve the isomerization of a double bond. Moore thought the approach had potential but recalls cautioning Qian, “The world doesn’t need just another mechanophore, we need better mechanophores.”
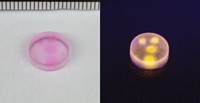
Qian and a team led by Moore and Sottos designed, built, and tested the new oxazine mechanophores (example shown), which they call OX. They demonstrated that OX was superior to the spiropyran mechanophore. OX changed color reversibly, in real time as the researchers repeatedly stretched and released the material (Chem 2021, DOI: 10.1016/j.chempr.2021.02.014).

Rint Sijbesma, who studies stimuli-responsive materials at Eindhoven University of Technology, points out that there are other fast and reversible mechanophores. But he says the OX mechanophore has broad potential because it could be possible to tune its color by changing substituents on its molecular skeleton and because it can be used in a range of polymers.
“The rapid timescale of switching, vibrant color change, and potential ease with which response can be tuned in the OX system are all promising and look to be improvements over existing systems,” says Stephen L. Craig, an expert on mechanophores at Duke University, in an email. “In the next decade, I think the impact of mechanochromic force probes like OX is certain to continue to grow.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter