Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Imaging platform illuminates biohybrids
Enzymes play different roles in decoupled and integrated biohybrids
by Celia Arnaud, special to C&EN
July 27, 2023
| A version of this story appeared in
Volume 101, Issue 25
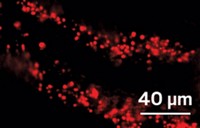
Biohybrid systems combine the light- harvesting capabilities of inorganic semiconductors with enzymatic synthetic capabilities of microorganisms. A new imaging platform that combines fluorescence microscopy with electrochemical mapping of light-induced currents provides new insights into biohybrid systems that use bacterial cells to produce the bioplastic polyhydroxybutyrate (PHB).
Peng Chen and coworkers at Cornell University used the new imaging platform to study so-called decoupled biohybrids and integrated biohybrids (Nat. Chem. 2023, DOI: 10.1038/s41557-023-01285-z). In decoupled biohybrids, semiconductors are used to electrolyze water and generate hydrogen gas, which is supplied to microbes. In integrated biohybrids, the microbes are attached to the semiconductors and take up photogenerated electrons directly from the material.
Ralstonia eutropha (also known as Cupriavidus necator) uses two hydrogenase enzymes—a soluble one and a membrane-bound one—to metabolize H2. The researchers used fluorescence labeling to visualize the hydrolases and cellular granules containing the PHB product. They found that both enzymes are important for electron uptake from photoexcited semiconductors in the integrated configuration and that the membrane-bound hydrogenase isn’t required in the decoupled configuration, but does play a facilitating role.
The researchers found that the current in individual cells in the integrated biohybrids reaches nanoamperes, which is “about 1,000 times larger than the picoamps that people would have expected,” Chen says. In addition, the researchers show that the electron transport pathway in the integrated biohybrids doesn’t involve H2 gas, which would be expected if electrolysis of water were involved.
“We found that the amount of electron current taken by the cell is very large,” Chen says. “The large current provides the opportunity, if you can rewire the electron pathways or biosynthetic pathways, maybe you can direct more of this current toward CO2 reduction instead of doing something else.”
Shelley D. Minteer, an expert on bioelectrocatalysis at the University of Utah, says the work is exciting because it provides a way to study and improve electron transfer in biohybrids. “This will make biohybrids (interfacing microbes with selective enzymes) for synthesis with (photo) electrodes easier to design and move forward to commercialization for interesting synthetic systems like bioplastics,” she writes in an email.
“The most exciting observation is the relatively large cathodic electron uptake by these microorganisms,” says Peidong Yang, an expert on photosynthetic biohybrids at the University of California, Berkeley.
Chen thinks the imaging platform will help study other combinations of semiconductors and microbes. “The imaging platform and also the way that we analyze the data, the analytical framework, the way we think about the results can be applied broadly to many kinds of these hybrid systems,” he says. These systems include ones that involve processes such as nitrogen reduction.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter