Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Protein-hydrogel crystal expands and contracts
Hybrid material could aid protein structure determination and drug delivery
by Stu Borman
May 3, 2018
| A version of this story appeared in
Volume 96, Issue 19

Shampoo plus conditioner, camera phones, and the spork are all common two-in-one products. In the same spirit of ingenuity, Akif Tezcan and coworkers at the University of California, San Diego, have now created a material that is not just a crystalline protein or an amorphous hydrogel but a hybrid of both (Nature 2018, DOI: 10.1038/s41586-018-0057-7).
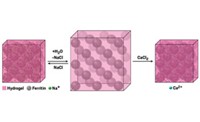
The hybrid can expand and contract several times without losing crystallinity. And if it fractures during expansion, it heals itself spontaneously, which crystalline materials typically cannot do.
“I have to admit that this paper is beautiful,” says porous materials specialist Christian Serre of École Normale Supérieure, in Paris. “It clearly bridges the giant gap between crystalline solids and soft matter by demonstrating that flexibility and crystalline order can coexist.”
The material’s ability to expand and contract repetitively could be useful to improve the quality of X-ray diffraction analysis of proteins, a feat the researchers demonstrated in their study. Such hybrid materials could capture, store, and release biomolecules like antibodies and nucleic acids for therapeutic and diagnostic use. And François Baneyx of the University of Washington predicts in a commentary accompanying Tezcan’s report that the material also has promise for sensors, separators, and actuators that have thus far been difficult to produce.
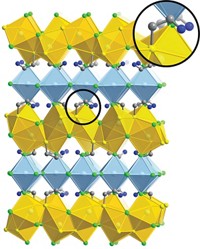
Tezcan and coworkers created the material by soaking crystals of human ferritin, an iron-storage protein, in a sodium chloride solution of hydrogel precursors. As the poly(acrylate-acrylamide) copolymer hydrogel forms, it integrates into void spaces in the protein crystal. The hydrogel and protein link to one another via ionic and hydrogen-bonding interactions, forming a true hybrid, not just a mixture.
Placing the material in deionized water depletes NaCl and causes the material to expand to nearly double its original linear dimensions and over five times its original volume. Adding NaCl again dehydrates the hybrid, contracting it to nearly its original size. Adding calcium chloride instead of NaCl promotes interactions between ferritin molecules even more effectively, returning the hybrid completely to its original dimensions.
X-ray diffraction of the CaCl2 hybrid demonstrated full recovery of crystalline periodicity and produced the highest resolution ferritin structure ever reported. “This suggests that the lattices of such hybrids are more precisely ordered than those of conventionally produced ferritin,” Baneyx writes. “Polymer infusion might thus be a useful approach to improve the quality of other protein structures or to access alternative structural states of proteins.”
The researchers believe the technique will be applicable to other proteins besides ferritin and note that diverse hybrids could be created by modifying and functionalizing the constituent proteins in various ways. They have applied for a patent on the technology.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter