Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Alternative materials could shrink concrete’s giant carbon footprint
Making cement releases 8% of the world’s anthropogenic CO₂. New formulations could slash that number
by Mitch Jacoby
November 22, 2020
| A version of this story appeared in
Volume 98, Issue 45

Credit: Shutterstock | Manufacturing the cement that binds the world’s concrete structures releases massive amounts of CO2 into the atmosphere.
On the list of materials people use most, concrete may be a runner-up, but it isn’t small potatoes. Second only to water in terms of how much is used across the globe, the ubiquitous construction material is produced in staggering quantities. According to industry analysts, some 30 billion metric tons of concrete is used globally each year to build bridges, roads, highways, high-rise buildings, sewage systems, and more.
In brief
Produced annually at the giga–metric ton level, cement serves not only as the glue that binds concrete but also as the stuff that holds together much of the modern world—bridges, dams, skyscrapers, and hidden infrastructure. But worldwide manufacture of this vitally important material releases some 8% of global anthropogenic CO2 emissions. As a result, researchers want to come up with alternative cement formulations and procedures that rein in these greenhouse gas emissions. These approaches, some of which are now being commercialized, include using waste products from other industries and nonstandard cement components that do not undergo CO2-emitting reactions during manufacturing.
Without a doubt, the hard-as-rock material supports much of the modern world. It’s also responsible for a lot of the world’s greenhouse gas emissions. The high-temperature process for manufacturing cement, the all-important glue that binds the components of concrete, accounts for roughly 8% of the world’s anthropogenic carbon dioxide emissions and consumes 2–3% of the global energy supply, according to the International Energy Agency.
“Concrete is a very useful material, and for that reason, we use a lot of it,” says Kimberly E. Kurtis, a civil engineer and concrete expert at the Georgia Institute of Technology. “We aren’t just using more concrete than ever,” Kurtis says. “We’re using more concrete per capita than ever,” she emphasizes, noting that global per capita consumption has nearly tripled in the past 40 years.
And the trend isn’t subsiding. As countries in Asia and Africa continue pushing forward with new construction projects, and nations in Europe and the Americas update aging infrastructure, demand for concrete continues to rise. Increased use could go hand in hand with greater environmental impact, unless manufacturers institute changes to the way this vital material is made.
Manufacturers have already implemented a number of changes. For example, as Ian Riley, CEO of the World Cement Association, an industry trade group, points out, by capitalizing on engineering advances, cement manufacturers have steadily improved the energy efficiency of the enormous kilns used for heating and processing the starting materials from which cement is made. Boosting energy efficiency reduces fuel consumption, which lowers CO2 emissions. Trapping those emissions also helps. Some cement makers are reducing net CO2 emissions through carbon-capture technology using solid sorbents or by sequestering the gas directly in concrete before it sets and solidifies.
A different way to tackle concrete’s CO2 problem is to reformulate cement with similar-behaving materials that inherently generate less CO2 than the ones used in traditional manufacturing methods. Another option is finding materials that enable manufacturers to use less of the CO2-generating components.
The push to reformulate cement isn’t new. But because of the growing threat of climate change, interest in the topic has intensified. Scientists in industry and academia are evaluating these alternative materials from top to bottom to determine if they are readily available and cost effective, are compatible with standard equipment and practices, and form products that are strong and durable.
What are cement and concrete?
Cement and concrete by the numbers
~8%:
Portion of global anthropogenic CO2 attributed to cement manufacturing
~30 billion metric tons:
Amount of concrete manufactured globally annually
1,450 °C:
Temperature of kilns used to process cement
25–50%:
Projected global increase in demand for concrete by 2050
Source: International Energy Agency; Nat. Mater. 2017, DOI: 10.1038/nmat4930.
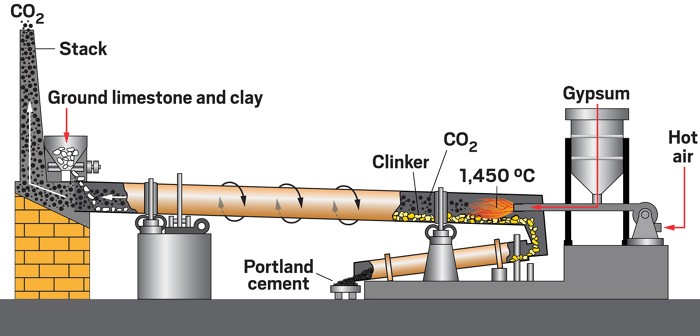
The standard material made by today’s cement companies is known as portland cement. The procedure for making it was developed in England about 200 years ago and has not changed much since then. The name comes from the resemblance of the hardened material to stone quarried on the Isle of Portland in England.
The method calls for heating powdered limestone, a widely available, inexpensive material, and clay to roughly 1,450 °C in a kiln. The high-temperature process, known as calcination, converts calcium carbonate (CaCO3), the principal component of limestone, to calcium oxide (CaO), or lime, releasing CO2.
That simple reaction, which is key to making cement, is the source of about half the CO2 emissions in cement manufacturing and a major reason why reducing emissions is challenging, says the Ohio State University’s Lisa E. Burris, a cement specialist and civil engineer. The bulk of the other half of the emissions comes from burning fossil fuels—often coal—to heat the kiln.
To produce cement, manufacturers grind the product of the calcination process, known as clinker, and mix it with gypsum, a calcium sulfate mineral, to prevent the powder from clumping and to control the subsequent reaction with water. Overall, the process emits more than 800 kg of CO2 for every metric ton of cement produced.
To make concrete, suppliers blend cement powder with sand and gravel and mix it with water, triggering a complex set of chemical reactions that convert the pasty mass to the strong, rock-hard solid so widely used in construction. One of the main reactions is the hydration of tricalcium silicate in cement, an exothermic process that releases calcium and hydroxide ions and forms calcium silicate hydrate, a phase that endows the synthetic rock with strength. A similar reaction with dicalcium silicate also strengthens concrete.
Cement 101
Waste opportunities
One way to lower emissions from making cement is to reduce the amount of limestone used by mixing it with a cement-like material that does not emit CO2. Fly ash, the powdery by-product of coal combustion that is typically rich in silica, alumina, and iron oxide, is a key example. It’s an inexpensive one, too. When mixed with water, it forms products with cement-like properties.

Years of research have shown that fly ash can decrease concrete’s carbon footprint while increasing its strength and workability—the ease with which it flows into place—Burris says. For some applications—for example, when rapid setting time is not required—portland cement can be blended with up to 40–50% fly ash, reducing emissions by roughly the same amount—and reducing cost.
Not only does fly ash help on the cradle end, meaning at the manufacturing stage, but it also helps on the grave end, by making concrete more durable, Georgia Tech’s Kurtis says. Extending the material’s lifetime reduces the emissions and energy used when making new cement to repair or rebuild a structure.
And, in principle, there’s plenty of fly ash lying around. Decades of accumulation from coal-fired power plants have resulted in enormous holding ponds filled with the material, enough to supply the industry for more than 130 years at current use levels, Burris says. What’s more, people living around coal plants want this waste removed because in some cases, it has caused major environmental contamination.
Yet for several reasons, fly ash ready to be added to cement is in short supply. For starters, the number of coal-fired power plants continues to decline relative to ones fueled with natural gas, so the quantity of fly ash produced is also decreasing. And because the chemical composition of fly ash varies with the type of coal from which it was derived and with specifics of the combustion process, not all the fresh material from a coal plant fulfills cement industry requirements or meets standards established by agencies such as ASTM International. For example, fly ash with a high carbon content (above 10%) can lead to porous concrete that is weak because of trapped air pockets.
Using the stuff recovered from long-term storage ponds isn’t straightforward either. That ash may have been commingled with other coal combustion by-products or undergone chemical changes during decades-long storage, which could interfere with cement hydration. “There’s a tremendous opportunity to use that fly ash in concrete,” Kurtis says, “but we need to understand how to use it effectively.”
Burris, Kurtis, and other researchers are systematically evaluating the range of chemical and physical properties that fly ash needs to have, and the extent to which it can be mixed with portland cement, to guarantee good performance. The material is already used in commercial concrete, but these researchers aim to broaden its use. They are working to update fly-ash standards to ensure that industry is on board with using reclaimed material and that the concrete products it yields perform well for decades.
Fly ash isn’t the only waste product making its way into cement and concrete. Cement makers also use blast-furnace slag, the calcium aluminosilicate by-product of smelting iron ore during steelmaking. As is the case with fly ash, mixing the powdered slag with water sets off reactions that form products with cement-like properties but does not emit CO2. So blending slag with portland cement reduces the fraction of the CO2-liberating component in cement, thereby lowering greenhouse gas emissions.
Some manufacturers—for example, Cemex, a multinational company headquartered in Mexico—offer commercial concrete products containing slag and fly ash. Davidé Zampini, Cemex’s head of R&D, notes that compared with traditional cement, Cemex’s Vertua Classic and Vertua Plus lines provide substantial CO2 reductions by substituting portland cement with these waste products at up to 30 and 60%, respectively.
But there aren’t enough of these by-products to meet the concrete industry’s giant demand, says Frank Winnefeld, a group leader in cement chemistry at the Swiss Federal Laboratories for Materials Science and Technology (Empa). So Winnefeld’s group is looking elsewhere.
In one out-of-the-box example, the Empa group teamed up with a European company that uses a metallurgical process to recycle cellular phones, a source of electronic waste that’s sure to continue growing. Stripped of precious metals, the residue contains calcium, silicon, aluminum, and iron and functions as a CO2-free cement binder, Winnefeld says. The team is now studying how to best use this material in cement formulations.
Green alternative cements
To find ways to reduce the carbon footprint of concrete, scientists have studied many types of cement in addition to the traditional portland type. Some of these alternative cements, including one based on heated clay, have garnered substantial attention.
Clays become active cementitious materials by heating, or calcining, them to around 800 °C, significantly lower than the 1,450 °C needed to produce ordinary cement, explains Karen Scrivener, a research group leader at the Swiss Federal Institute of Technology, Lausanne (EPFL). Not only does calcining clay use less CO2-emitting fuel, but it also does not involve decomposing limestone, a major source of the gas in traditional cement preparation. The clay-based cement includes some limestone, but it’s not heated, Scrivener notes.

The product, limestone calcined clay cement, or LC3, is blended with portland cement and has roughly the same properties as the more traditional material, Scrivener says. But LC3’s carbon footprint is 40% smaller than that of traditional cement. Kurtis, who is also a proponent of LC3, notes that it can be produced worldwide using impure clay, making it practical and economical.
LC3 is starting to make inroads. “We have done full-scale trials in India and Cuba,” Scrivener says. In addition, an LC3 product was just commercialized in Colombia, she notes, and a full-scale calcining plant is being commissioned in Côte d’Ivoire.
In the alphabet soup of cement types, CSA, calcium sulfoaluminate, also draws a lot of attention for its environmental friendliness. Compared with standard cement preparation, CSA processing emits less than half the CO2 (less than 20% in some cases) because the level of limestone in the raw material mix is lower and the working temperature is 200 °C cooler.
CSA cements, which are used commercially for a limited number of applications, harden much faster than the standard portland type, and they tend to be more costly, Winnefeld says. The fast-setting nature makes them ideal for repair work but unsuited to many types of construction.
He notes, for example, that these materials are used at Zurich Airport for overnight runway repairs, when there is a brief lull in airplane traffic. And Burris tells a story about a sinkhole that opened during morning rush hour on an Illinois highway and was patched with CSA cement in time for evening rush hour. “That would have been impossible with a portland cement,” which often requires several days to set firmly, Burris says. Researchers are studying ways to slow CSA’s hydration kinetics to enable the ecofriendly cement to be used more broadly.
Geopolymers are another category of low-CO2-emitting cements that are making a splash in the news. Cemex just launched Vertua Ultra Zero, a geopolymer clinker-free concrete, in which an alkali-activated alumina-silicate matrix serves as the binder, according to Cemex’s Zampini. The material does not require high-temperature processing, and it reduces CO2 emissions by 70% relative to traditional cement.
Cement at the molecular level
To tweak cement formulations to make them more environmentally friendly, scientists also need to better understand what cement does at the molecular level. But when it comes to cement chemistry, Zampini is fond of saying, “It’s not rocket science; it’s rock science.”
That saying may seem to suggest that the basics are simple and were established long ago. But they aren’t, and they weren’t. Decades after researchers began tackling the central hydration reaction, which imparts strength to cement and concrete, many of the details remain unknown. Two recent studies highlight the way scientists are using powerful analytical methods to ferret out elusive details.
Advertisement
In one case, Oklahoma State University’s Masoud Moradian and colleagues at Princeton University and Argonne National Laboratory used X-ray tomography methods to track the nanoscale evolution of 60,000 particles in a sample of portland cement during the first 16 h of hydration. Analyses showed that larger particles tended to grow by accumulating minerals containing the sample’s heavier elements, while the surfaces of smaller particles steadily dissolved (Constr. Build. Mater. 2019, DOI: 10.1016/j.conbuildmat.2019.04.013). A better understanding of this process could lead to improvements in concrete durability, Moradian says.
In the other study, a team of researchers including EPFL scientists Scrivener, Aslam Kunhi Mohamed, and Lyndon Emsley combined computational methods and nuclear magnetic resonance spectroscopy to determine the atomic-level structure of the calcium-aluminate-silicate-hydrate phase typical of many low-CO2 cements (J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.0c02988).
The way that strength and other cement properties develop depends on this phase, Scrivener says. “For a long time, its structure has been a mystery.” Understanding the way it grows, she says, “is the secret to going further with reducing cement’s environmental impact.”
With billions of metric tons produced every year, concrete is certainly the stuff of the modern world. “It’s a fascinating material, and it’s vital for society,” Kurtis says. But its greenhouse gas emission problem needs to be tamed. “We can’t do without concrete,” Scrivener says. “But we can do without a significant amount of the emissions it produces.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter