Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Chromium ions can form clusters in molten salts
Unexpected finding deepens understanding of corrosion in molten-salt nuclear reactors
by Mitch Jacoby
May 27, 2021
| A version of this story appeared in
Volume 99, Issue 20

Highly dilute metal ions can aggregate in a high-temperature ionic-liquid medium and form long-lived clusters. The unexpected finding, which is the result of a study that combines experiment and modeling, sheds light on the early stages of corrosion in molten-salt nuclear reactors (MSRs).
MSRs are a next-generation, high-efficiency nuclear power plant technology in which nuclear fuel contained in a high-temperature salt circulates through the reactor in the liquid state. By running at roughly 500 °C higher than today’s solid-fuel commercial reactors, MSRs promise to boost thermodynamic efficiency.
More than 50 years after researchers began studying MSRs, however, the technology remains under development. One reason is that molten salts can corrode reactor vessels, slowly leaching chromium from alloys that otherwise perform well.
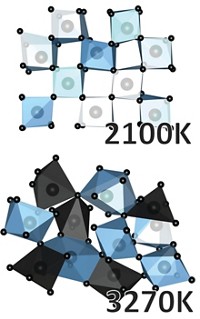
To probe the corrosion process at the atomic level, Alexander S. Ivanov of Oak Ridge National Laboratory and coworkers used X-ray scattering and theoretical simulations to study the behavior of chromium ions in a mixture of potassium chloride and magnesium chloride at up to 1,073 K. They found that the metal forms long-lived chloride-bridged chromium clusters, which was unexpected because of the high temperature and the ions’ low concentration (Chem. Sci. 2021, DOI: 10.1039/D1SC01224J).
The results, which could provide insights into how to stop the corrosion of the alloy by molten salts, challenge the paradigm of molten salts simply being a sea of cations in a sea of anions, Ivanov says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter