Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
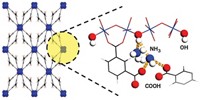
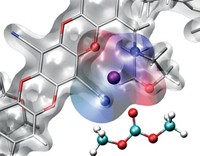
Metal-Organic Frameworks
Cu-based material shows promise for safer battery electrolytes
MOF could be used in solid-state batteries
by Mitch Jacoby
March 2, 2019
| A version of this story appeared in
Volume 97, Issue 9
Lithium-ion batteries power many of today’s electric vehicles and nearly all portable electronics because they cram a lot of energy into small, lightweight packages. But they depend on flammable liquid organic electrolyte solutions to shuttle ions between the electrodes, and those liquids pose a small but potentially serious fire hazard. Scientists have been examining nonflammable solid electrolytes as alternatives. Most of the ones tested have ion conductivity values too low for practical use. Furthermore, the materials leave little room for improvement via chemical customization. To address these shortcomings, the Massachusetts Institute of Technology’s Elise M. Miner, Sarah S. Park, and Mircea Dincă developed a method for loading lithium, magnesium, and aluminum halides into a copper-based metal-organic framework (MOF), which can be tuned by altering the organic linkers. The team found that the MOFs exhibit high ionic conductivity for Li+ and roughly record-tying values for Mg2+ (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.8b13418). Optimizing the size and polarity of MOF pores may lead to inherently safe magnesium-ion batteries that benefit from twice as much charge-capacity associated with divalent ions, the team notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter