Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
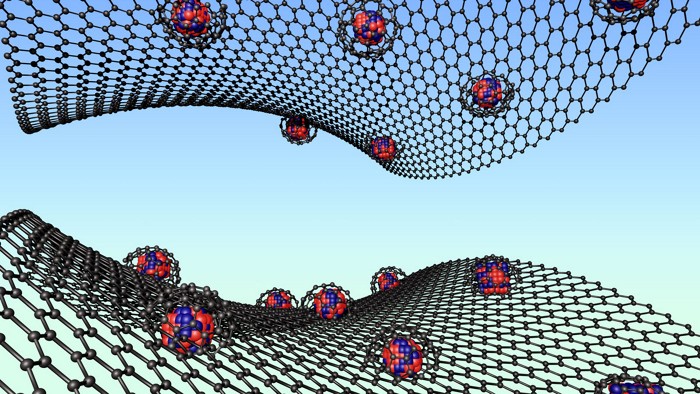
Metal-Organic Frameworks
MOF catches xenon and krypton
Porous material could trap radioactive gases released during nuclear fuel treatment
by Mark Peplow, special to C&EN
February 13, 2022
| A version of this story appeared in
Volume 100, Issue 6

A porous metal-organic framework (MOF) material that sucks up record amounts of xenon and krypton could offer an energy-efficient way to capture those gases during nuclear waste processing (Angew. Chem., Int. Ed. 2022, DOI: 10.1002/anie.202117807). Traces of radioactive xenon and krypton contaminate oxygen, nitrogen, and other gases released during nuclear reprocessing. Expensive cryogenic distillation is typically used to remove these isotopes and ensure they don’t escape into the environment. Researchers now have found that a MOF identified 20 years ago (J. Am. Chem. Soc. 2000, DOI: 10.1021/ja003159k) can grab more xenon and krypton than other porous materials. The MOF is a lattice of copper ions connected by adamantane tetracarboxylate ligands. Its pores are lined with C–H groups that preferentially bind the two noble gases, and some of them can shrink to fit perfectly around the gas atoms, says team member Shengqian Ma of the University of North Texas. The researchers tested the MOF with a mixture of oxygen and nitrogen containing 400 ppm xenon and 40 ppm krypton, a composition typical of the gas released during nuclear reprocessing. At atmospheric pressure and 25 °C, the MOF’s capacity for xenon was 32 mmol/kg of material, almost three times as much as the previous record holder, and its krypton capacity reached a new high of 8 mmol/kg. “In terms of the overall performance, it could replace cryogenic methods,” Ma says. The team is now trying to reduce the cost of the ligand and produce the MOF at kilogram scale, potentially using a flow reactor.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter