Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Metal-Organic Frameworks
Metal-organic frameworks could cut chiral chromatography costs
The versatile MOF-filled column separates enantiomeric drug compounds by high-performance liquid chromatography
by Tien Nguyen, special to C&EN
September 21, 2019
| A version of this story appeared in
Volume 97, Issue 37

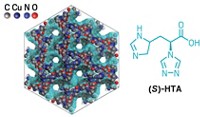
In drug discovery, separating enantiomers of a compound usually involves high-performance liquid chromatography (HPLC) columns packed with specialized chiral stationary phases. These columns are usually compatible with only polar or nonpolar solvent mixtures, meaning chemists sometimes have to switch between columns to find the right solvent system. Now, a team of researchers has demonstrated a reusable and scalable metal-organic framework (MOF) that can do chiral separations with both polar and nonpolar solvents (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b06500). José Ramón Galán-Mascarós of the Institute of Chemical Research of Catalonia and colleagues synthesized a water-stable MOF composed of copper atoms linked with modified l-histidine molecules. Galán-Mascarós’s lab teamed up with Melissa M. Reynolds of Colorado State University and other collaborators and showed that an HPLC column packed with this MOF could separate the enantiomers of the pain-relieving drug ibuprofen and the infamous medication thalidomide, which was used to treat morning sickness until scientists discovered that one of its enantiomers could cause birth defects. They also found that the column performed as well as three commercial columns at separating a reference racemic mixture. Using the MOF-packed column, however, researchers could switch among solvent systems simply by flushing the column with the appropriate solvent.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter