Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomaterials
Biphenylene network zipped together
Flat carbon allotrope assembled using HF-zipping reaction
by Bethany Halford
May 21, 2021
| A version of this story appeared in
Volume 99, Issue 19

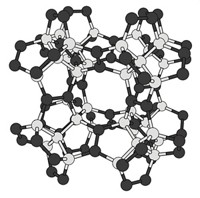
In the world of flat carbon allotropes, graphene gets most of the attention. But there are other ways of assembling sp2-hydridized carbon frameworks; they’re just challenging because of this type of carbon’s tendency to form benzenoids, structures formed exclusively from benzene rings. Scientists led by J. Michael Gottfried of Philipps University of Marburg and Peter Liljeroth of Aalto University, now report the synthesis of a biphenylene network—a planar structure composed of sp2-hybridized carbons in 4-, 6-, and 8-membered rings (Science 2021, DOI: 10.1126/science.abg4509). The researchers created the biphenylene network (shown) by zipping together strings of 2,5-difluoro-para-phenylene on a gold surface using a dehydrofluorination reaction. This hydrogen-fluorine (HF)-zipping strategy, they note, could be used to assemble other nonbenzenoid carbon allotropes. Theoreticians had disagreed about the possible properties of a biphenylene network: some argued that it would be metallic, while others postulated it would be dielectric. Scanning probe studies of the allotrope indicate that it is metallic. This means that it could be used to make wires for carbon-based circuits in the future, the scientists say.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter