Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Polymers
Modified adsorbent with conductive polymer grabs uranium from seawater quickly
New material could help extract oceans’ vast but dilute supply of nuclear fuel
by Mitch Jacoby
May 17, 2020
| A version of this story appeared in
Volume 98, Issue 19

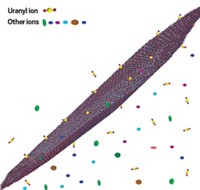
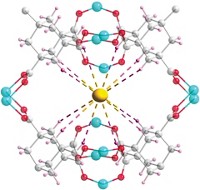
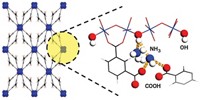
Earth’s oceans contain more than 4 billion metric tons of dissolved uranium. That’s roughly 1,000 times as much as all known terrestrial sources combined, and enough to keep the world’s nuclear power plants running for centuries. But because the oceans are enormous and uranium’s concentration in seawater is so low—roughly 3 ppb—extracting the metal is a tough job. A new adsorbent featuring conductive polymer chains may make that task easier (Chem 2020, DOI: 10.1016/j.chempr.2020.04.012). Ye Yuan and Guangshan Zhu of Northeast Normal University and coworkers sought to improve the uranium uptake properties of a promising porous aromatic material, MISS-PAF-1, that they had used in earlier studies to extract uranium via an electrochemical method. That material traps uranyl ions at adsorption sites containing salicylaldoxime groups. To boost the performance, the researchers threaded MISS-PAF-1’s channels with poly(phenylacetylene), reasoning that the conductive chains would provide a pathway for ion transport and concentrate uranyl ions near adsorption sites. Tests on natural seawater show that modified MISS-PAF-1 extracts uranium more than three times as fast as the unmodified adsorbent and several reference adsorbents, trapping 13 mg of uranium per gram of adsorbent in 56 days.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter