Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nobel Prize
Lithium-ion battery pioneers nab 2019 Nobel Prize in Chemistry
John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino will share the prize for developing the chemistry of the rechargeable batteries
by Bethany Halford
October 9, 2019

The 2019 Nobel Prize in Chemistry has been awarded to John B. Goodenough of the University of Texas at Austin, M. Stanley Whittingham of Binghamton University, and Akira Yoshino of Asahi Kasei Corporation and Meijo University “for the development of lithium-ion batteries.” The three will share the roughly $1 million prize equally.
“The lithium-ion battery is shaping the modern world in ways that couldn’t have been anticipated when it was first discovered,” says Gavin Harper, a research fellow at the Faraday Institution and an expert in renewable energy at the University of Birmingham. “It’s an enabling technology that has made possible Star Trek-like devices that we have in the modern world, from smart watches to phones.”
Lithium-ion batteries provide a lightweight, rechargeable power source for mobile phones, laptop computers, and electric vehicles. Unlike conventional batteries, which get their power from chemical reactions that break down the electrode, lithium-ion batteries generate power via the reversible flow of lithium ions between the anode and the cathode. But to make this technology possible, chemists had to tame lithium—an alkali metal that’s prone to explosion.
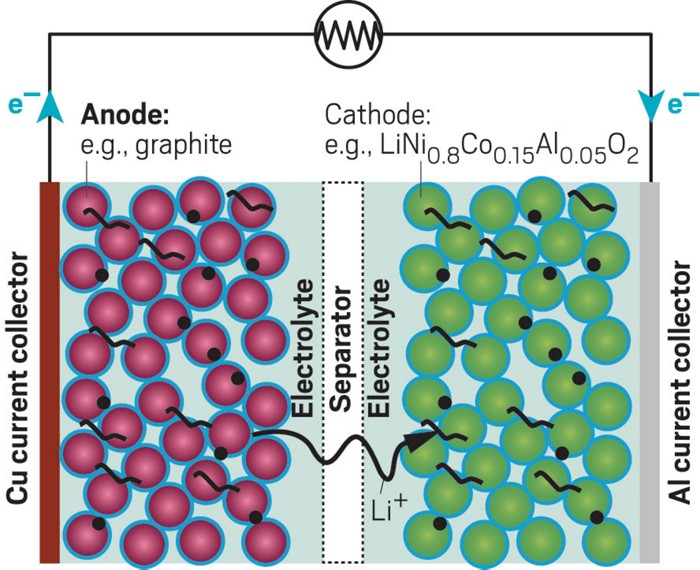
The story of lithium ion batteries’ discovery dates back to the 1970s, during the decade’s oil crisis. Back then Whittingham, who was working for the oil and gas company Exxon, was researching energy-rich materials when he figured out how to make a battery cathode from titanium disulfide (TiS2). TiS2 is a layered material, and lithium ions slip between its layers—a process known as intercalation. Whittingham paired this with an anode made from metallic lithium and added an organic liquid electrolyte that could conduct lithium ions between the two electrodes. This was the first rechargeable lithium battery.
But the battery was not without its flaws. The lithium metal could form whispy needles that caused the battery to short circuit, overheat, and then, possibly, explode. Goodenough, who was working at Oxford University, discovered that lithium ions could also intercalate through cobalt oxide. At around the same time, Yoshino, who was working at Asahi Kasei Corp., showed that lithium ions could also intercalate in petroleum coke.
By using cobalt oxide as the cathode and petroleum coke as the anode, the researchers had a battery that could run at about 4 V, much higher than the about 2.4 V battery Whittingham developed. It also was safer because it contained no metallic lithium. The battery could be recharged hundreds of times without its performance deteriorating and it was introduced commercially in 1991.
Recognition of these chemists’ work with the Nobel Prize has been anticipated by chemists for many years. At 97, Goodenough is the oldest Nobel laureate ever. “There were a lot of scientists that were really rooting for Goodenough this year,” Harper says.
Olof Ramström, a member of the Nobel Committee for Chemistry, couldn’t resist a battery pun as he explained the trio’s work during a press conference this morning, calling it “a highly charged story of tremendous potential.”
American Chemical Society (ACS) President Bonnie Charpentier says that this year’s Nobel announcement is “particularly exciting because the invention of the lithium-ion battery is something that everyone around the world can see the importance of—it enables transportation, it enables communication, it enables storage and portability of energy that makes people more efficient and safer.” She adds, “It’s really exciting to see something that recognizes collaborative work, building on one piece of work after another from three of our long-term members. Among them they have more than 100 years of membership in ACS.” C&EN is published by ACS.
Harper also thinks the prize is timely. “We sit at this crossroads where there’s heightened awareness of the impact we’re having on the world and the need for decarbonization,” he says. “It seems as if those efforts are reaching a crescendo, so I think it’s really timely that this technology that really sits at the nexus of so many other technologies and enables decarbonization should come up this year.”
CORRECTION
This story was updated on Oct. 10, 2019, to correct the anode material Yoshino worked with—petroleum coke, not graphite—and to clarify the voltage of Whittingham's first battery.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter