During the oil crisis of the 1970s, a chemist at Exxon named M. Stanley Whittingham, working on a new type of rechargeable battery, discovered that lithium ions could slip inside the gaps in a layered material called titanium disulfide. He created the world’s first lithium-ion battery, which sported a titanium disulfide cathode and a lithium anode. The device was lightweight and had excellent energy storage capacity, but it frequently short-circuited. John B. Goodenough, then at the University of Oxford, and Akira Yoshino at Asahi Kasei refined the design by switching out the titanium disulfide for cobalt oxide and the lithium metal for petroleum coke. The trio won the 2019 Nobel Prize in Chemistry for the work.
Just 50 years after Whittingham’s original invention, lithium-ion batteries have come to power an enormous swath of our world. Our cell phones, laptops, power tools, and electric vehicles all rely on this technology, and demand is now expanding to larger-scale energy storage for electricity generated by solar cells and wind turbines.
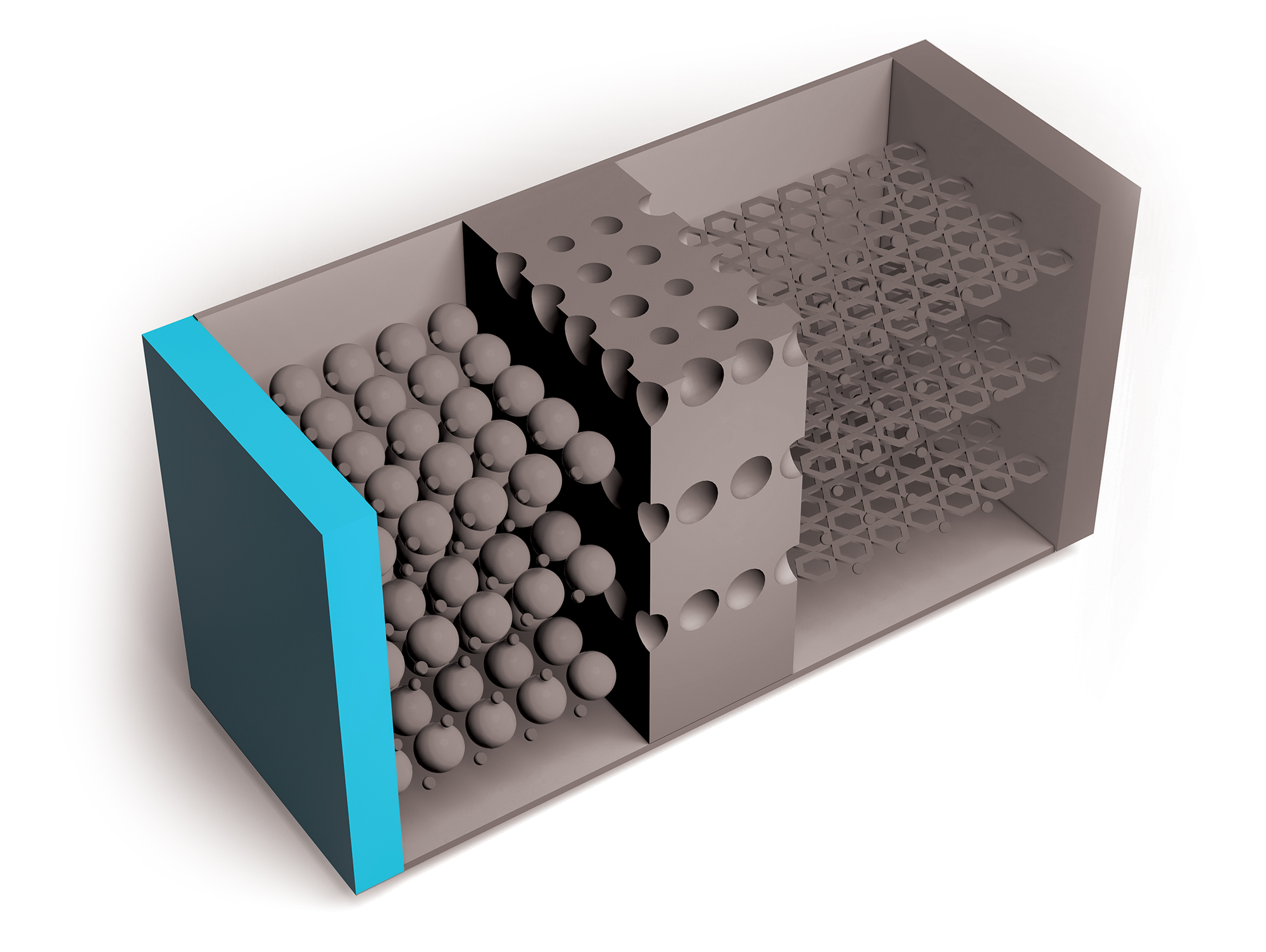
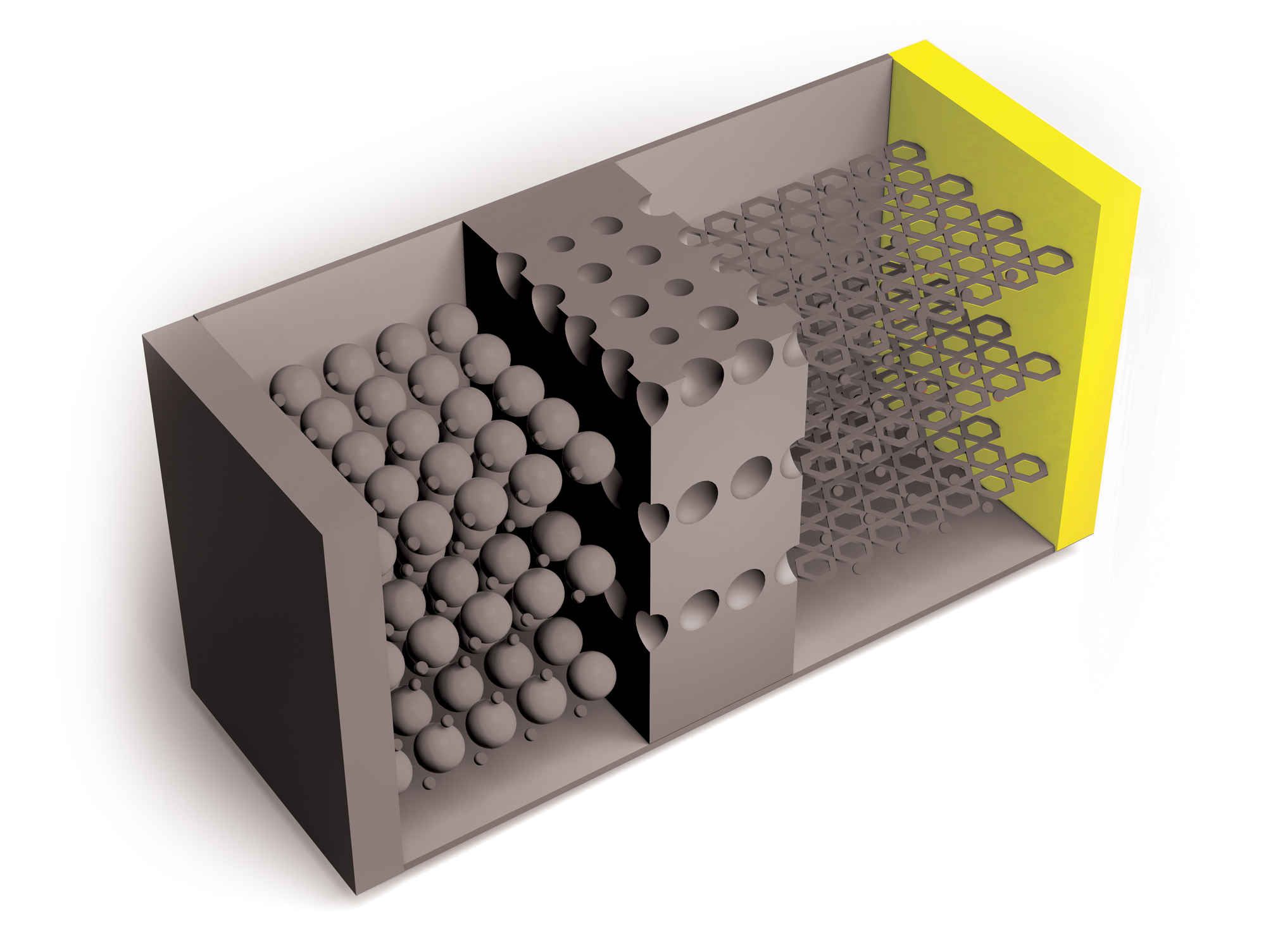
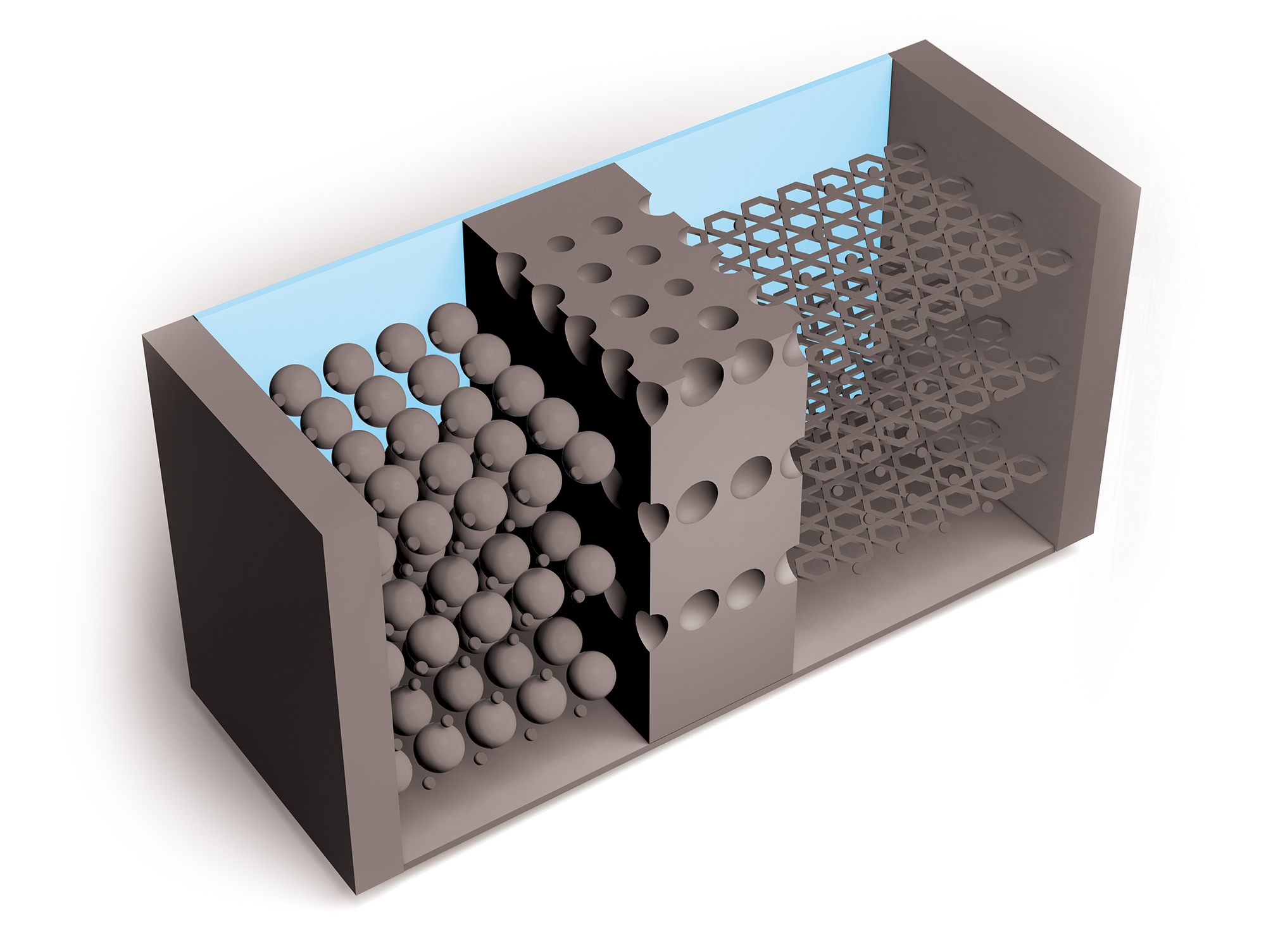
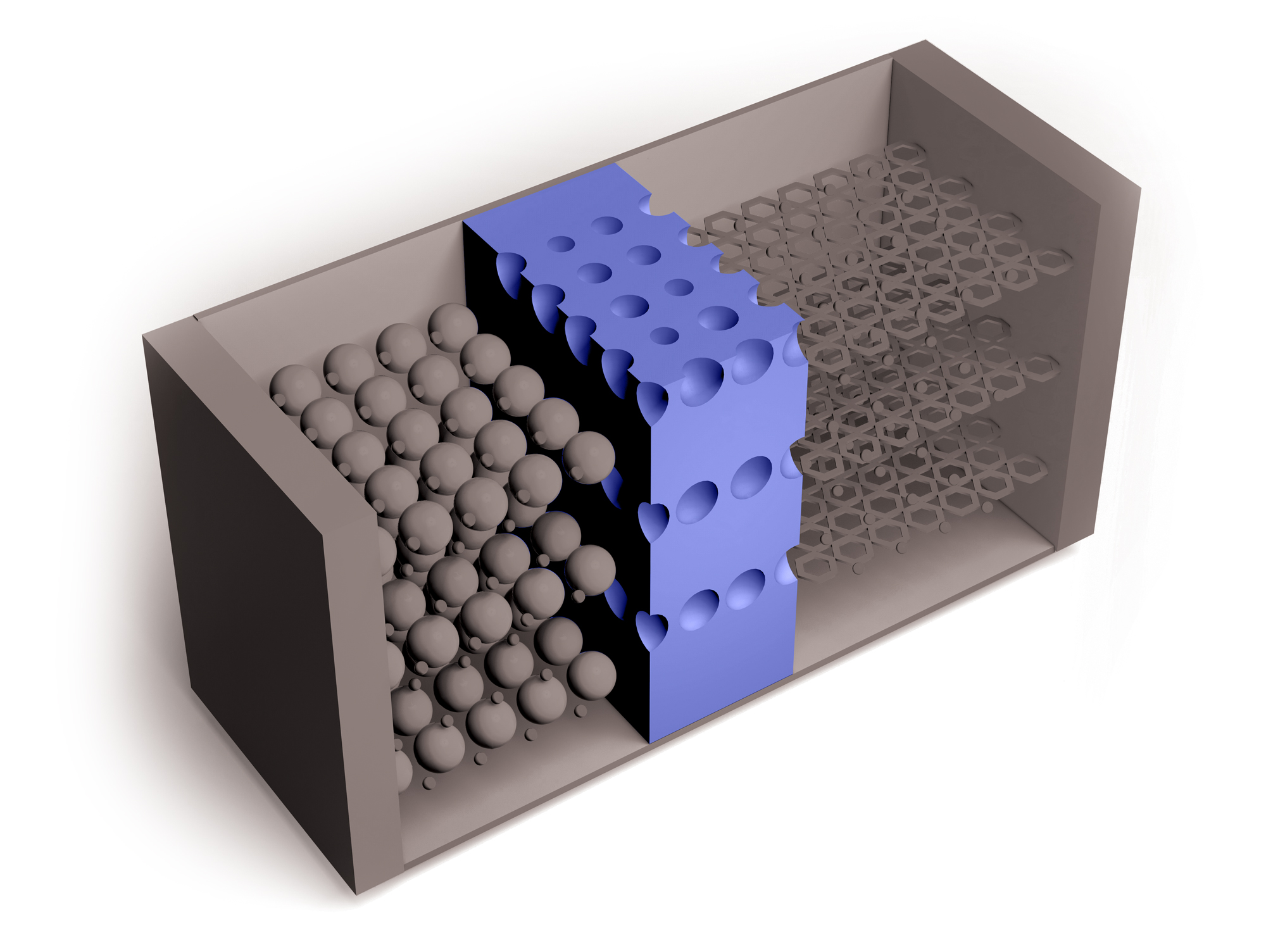
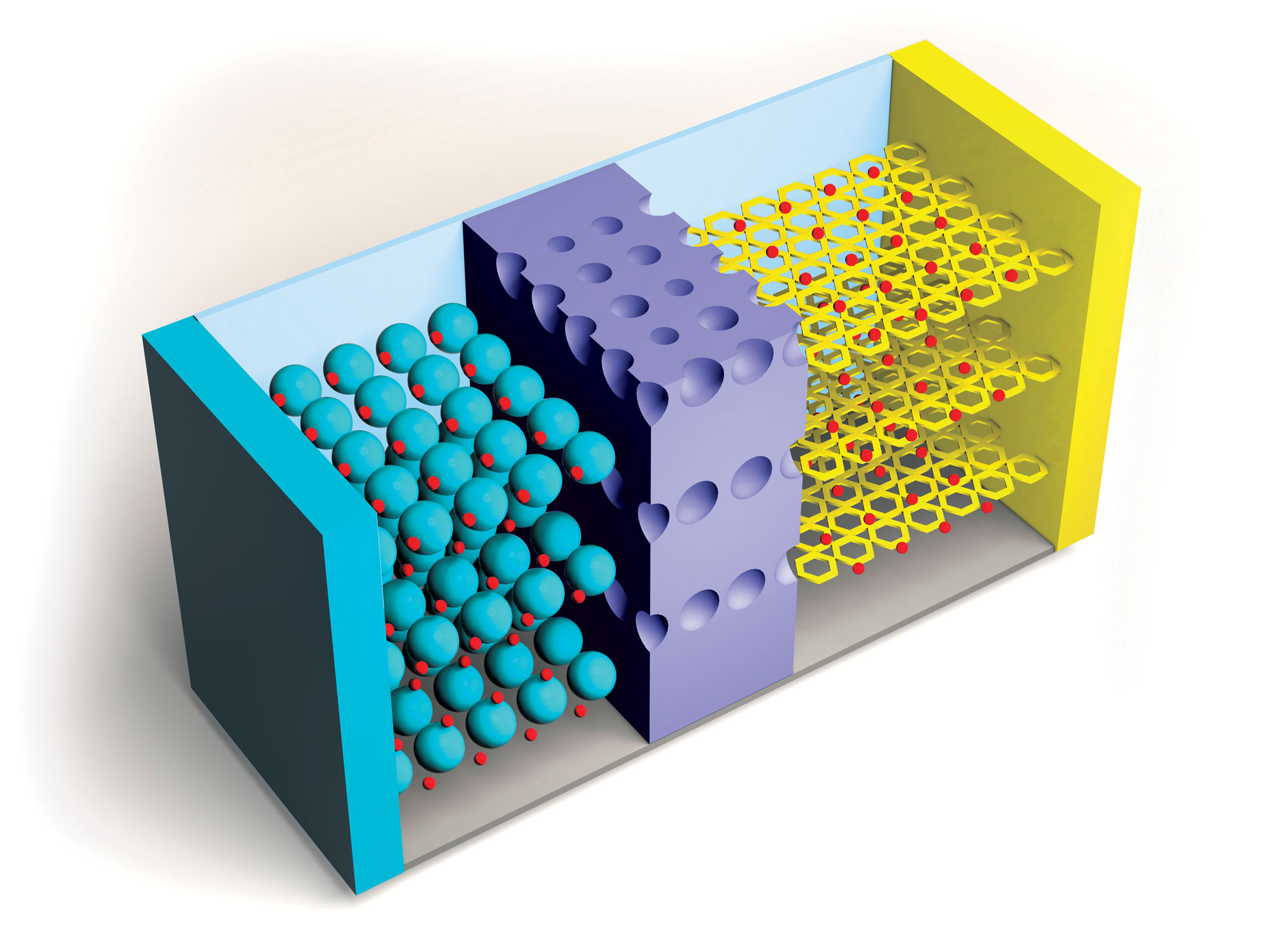
Researchers continue to improve on Li-ion batteries’ four key components: the cathode, anode, electrolytes through which lithium ions travel, and separators that keep positive and negative electrodes apart. But the need for higher capacity and safer and cheaper power sources is growing, as is the recognition of environmental harms caused by mining metals required for Li-ion batteries.
Spurred by this reality, researchers are exploring a wide array of chemistries and materials for batteries beyond lithium ion. One approach gathering momentum is sodium-ion batteries, which originally gained interest in the 1980s, before lithium ion became the dominant technology.
“There’s a whole new chemistry and electrochemistry that has to be developed for sodium,” says Linda Nazar, a chemist at the University of Waterloo. For now, this technology’s relatively low energy density makes stationary energy storage the most obvious application, but that could change as new research brings improvements, she says. Researchers are investigating other ions, such as magnesium and sulfur, as well as changes in cathode, anode, and electrolyte materials, but most of these approaches have a way to go before commercialization.
Whichever battery chemistries and materials end up on top, the industry will have to address the issue of battery recycling, which today is barely done. “As people get beyond the growing pains of things like electrifying cars, we face hard questions about where those spent batteries go,” says Lynden A. Archer, a chemical engineer at Cornell University. The next wave of power storage technology “could be the next big thing for society,” he says—but for it to be successful, governments, scientists, and policymakers will have to create frameworks for greening the industry.










Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter