Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biologics
Microbe-loaded contact lens lubricates itself
Engineered bacteria continuously pump out a wetting agent, providing an alternative to eye drops
by Carolyn Wilke, special to C&EN
May 3, 2024
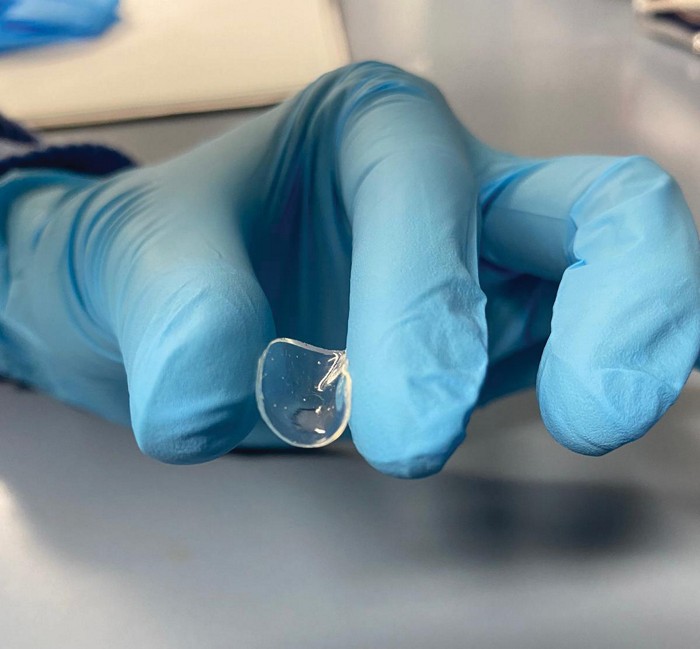
After a long day, contact lenses don’t usually feel comfy. And any relief offered by eye drops can fade quickly. Now researchers have developed “living” soft contact lenses that lubricate themselves—with molecules produced by microbes (Adv. Mater. 2024, DOI: 10.1002/adma.202313848). Someday a similar strategy might be used to deliver drugs to the eyes.
Contact manufacturers often impregnate lenses with wetting agents. “But of course, at some point, after a few hours, the contact lens is empty,” says Aránzazu del Campo, a biomaterials chemist at INM—Leibniz Institute for New Materials. Contacts’ capacity for lubricants is limited by the need to maintain their optical properties. And the eye’s secretion of tear fluids constantly clears away anything that is delivered. The residence time of lubricants or drugs provided by eye drops is brief—only a few minutes, she says.
But del Campo and her colleagues realized that microbes could pump out a continuous supply of lubricant. The researchers created soft contacts made of a poly(vinyl alcohol) hydrogel. At the outer edge of the lens, they embedded a transparent ring of bacteria-laden hydrogel. The team engineered Corynebacterium glutamicum bacteria, relatives of microbes that dwell around the human eye, to produce hyaluronic acid, a wetting agent.
The amount of hyaluronic acid released by the lenses reached the level recommended for therapeutic doses and could be tuned by tweaking the hydrogel’s mechanical properties, which controls growth of the bacterial population. In a test lasting 21 days—a time longer than typical usage for soft contacts—the lenses sustained production of hyaluronic acid.
These contacts showcase a new potential application of living materials, says Avinash Manjula-Basavanna, a synthetic biologist and materials engineer at Northeastern University, who was not involved with the work. “In the field of engineering living materials, what we try to do is . . . use living cells as factories to produce materials.”
Living contact lenses could also be used to administer drugs, del Campo says. Microbe-loaded lenses could deliver antibiotics or compounds that need to cross the cornea because the bacteria could boost levels of drugs in the eye better than other approaches. But first, governments, researchers, and companies would need to figure out how bacteria-filled lenses would be regulated, she says.
The research is fun and exciting, says Anuj Chauhan, a chemical engineer at the Colorado School of Mines, who wasn’t involved with the study. “But it’s a long way from potentially really getting to a point where you can actually commercialize it. And there are many, many challenges.” It may prove difficult to sanitize lenses without killing the lubricant-releasing microbes, to produce lenses infused with living biological agents as cheaply as current options, and to package and distribute living lenses before their microbes die.
But the researchers see some benefits over traditional approaches to delivering compounds to the eye. “In the context of sustainability, I think these lenses can make a huge difference,” del Campo says. A great deal of money is spent to make, purify, and package biopharmaceuticals, drugs made by living things. “Using this approach, we don’t have to produce it. We don’t have to isolate it. We produce it on site.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter