Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Oncology
Glycoscience fuels a new breed of cancer immunotherapy
Start-ups like Palleon and NextCure look to broaden the benefits of immuno-oncology with drugs that target a protein family called Siglecs and their glycan ligands
by Esther Landhuis, special to C&EN
July 20, 2019
| A version of this story appeared in
Volume 97, Issue 29

As a biochemist developing antibody therapies at a major pharmaceutical company, Li Peng spent years fixated on membrane proteins. But every now and then, she wondered if she and her colleagues were missing something. Proteins carry out a cell’s work, yet whether and where they act often depend on another type of biomolecule—glycans. These sugars that coat the surface of a protein can influence a drug’s structure and stability and even dictate if it works at all.
“In the back of my mind, I always felt we had overlooked glycobiology,” says Peng, who worked for nearly a decade at MedImmune, AstraZeneca’s biologics research arm. “But I didn’t have the resources or the tools or the power to make a big initiative.”
In the summer of 2016, Peng received an intriguing email from the CEO of a Boston-area start-up. He hoped to interest her in an entirely new approach to cancer immunotherapy.
Currently approved immunotherapies known as checkpoint inhibitors work by releasing the brakes on immune fighters called T cells, freeing them to kill tumors. These drugs have helped thousands of people whose cancers were once considered incurable. Their effect can be so dramatic for some cancers that the science behind the treatments won a 2018 Nobel Prize.

But checkpoint inhibitors work for only a fraction of the population—a recent analysis suggests that less than 13% of people with cancer respond to the drugs.
The new start-up, Palleon Pharmaceuticals, thought glycoscience might be the key to expanding the reach of cancer immunotherapies. The company wanted to target membrane proteins called Siglecs (sialic acid–binding immunoglobulin-like lectins), which suppress immune activity in much the same way as the receptors hit by current checkpoint blockers, with two notable differences. First, Siglecs adorn the surface of a wide range of immune cells, not just T cells. Second, Siglecs don’t recognize proteins. Rather, their ligands are sugars called sialic acids. The CEO, Jim Broderick, was looking for someone with Peng’s protein-engineering and drug-discovery experience to join his team.

Palleon is one of several companies going after Siglecs. Most firms are developing drugs that directly target Siglecs, but Palleon’s approach is distinct: its lead program targets the sugars that bind to Siglecs. Together, these biotech firms’ efforts are ushering in a new wave of glycoscience drug discovery in cancer. Already one drug has entered into clinical studies, and another is expected to enter human testing by early 2021.
A new checkpoint?
When Broderick contacted Peng, his company didn’t yet have a website and hadn’t yet secured significant funding. A year earlier, he didn’t even know what a Siglec was. SR One, GlaxoSmithKline’s venture capital arm, had hired Broderick to look for opportunities to start a company in the innate-immunity space. At first he focused on macrophages—cells that help form the immune system’s first line of defense. Macrophages shift their behavior according to environmental cues sensed through their surface receptors that recognize molecular structures found in microbes such as bacteria, viruses, and fungi.
Palleon Pharmaceuticals launched in July 2015 to develop drugs that could manipulate macrophages. Scouring papers on innate immune pattern recognition, Broderick learned that the vast majority of these surface receptors sense glycans. “It surprised me,” he says.
Many scientists forget that proteins on a cell’s surface sit beneath a dense glycan forest. Under a microscope, this foliage makes cells look fuzzy. Yet despite their ubiquity, sugars languish in relative obscurity. Sugars attach to proteins and lipids to form complicated biological structures that can’t be pared down into the neat, linear sequences used to represent DNA or proteins. Ajit Varki, a glycobiologist at the University of California San Diego, calls glycans “the dark matter of the biological universe.”
To brainstorm possibilities for Palleon, Broderick found key papers on glycan-binding receptors in innate immunity. He emailed authors asking to chat about their research. “Every time I’d call someone, I’d say, ‘Who else do you know in this field doing interesting work?’ ” he says. “And I started to build a network.”
Early chats with Siglec pioneers Paul Crocker at the University of Dundee in Scotland and James Paulson of Scripps Research in California left Broderick wondering if targeting Siglecs could shut down an overactive immune system—perhaps to treat fibrosis or autoimmunity.
Those ideas vanished when a phone call with Stanford University chemist Carolyn Bertozzi turned his attention to immune checkpoints in cancer.
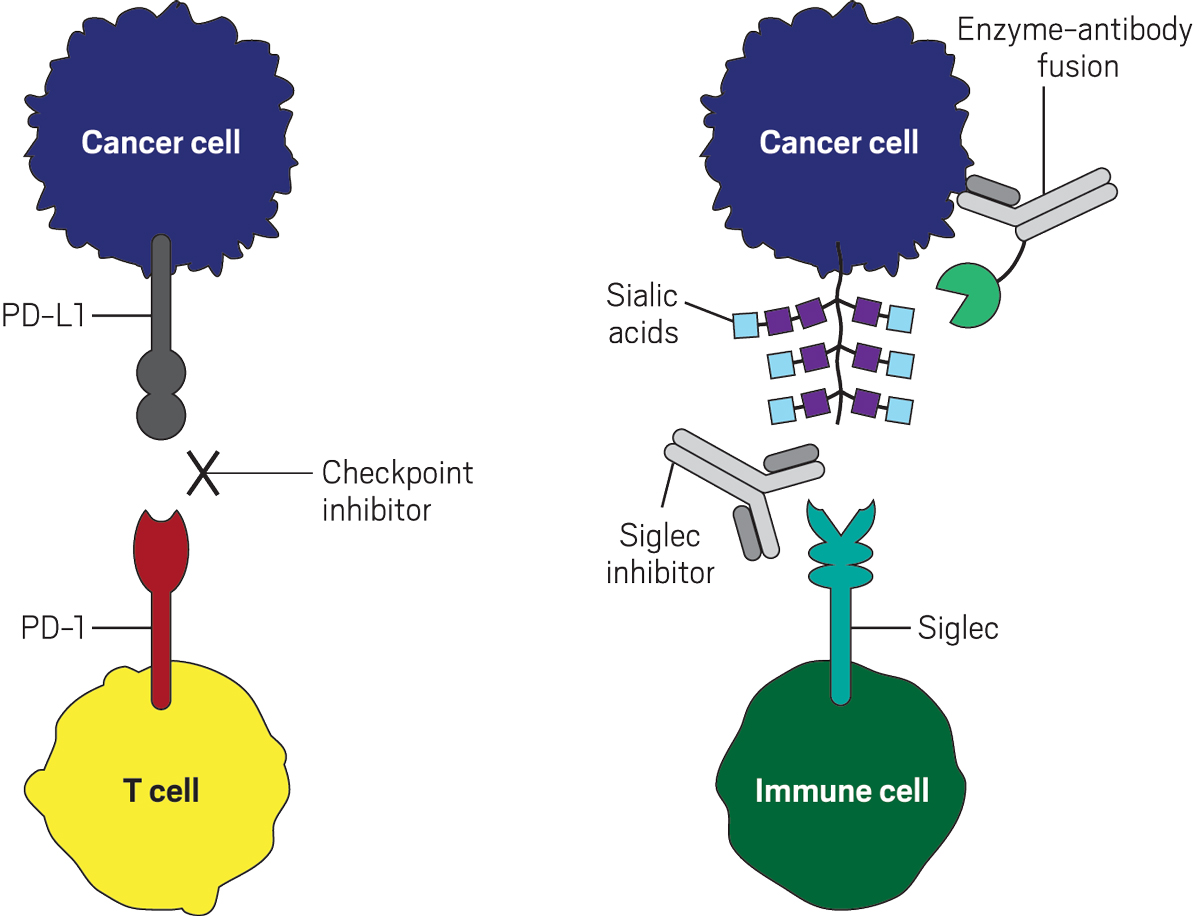
Scores of researchers have worked on checkpoint inhibitor drugs. The most widely used treatments block PD-1, a receptor that sits on the surface of killer T cells, while other approved drugs block its ligand PD-L1. Normally, killer T cells attack tumors. However, if a T cell’s PD-1 binds to PD-L1, which is overexpressed on the surface of some cancer cells, the T cell falls down on the job, and the tumor cell evades detection. In other words, PD-L1 molecules shield cancer cells from immune attack.
As it turns out, tumor cells also hide from the immune system by altering their glycan coat (Nat. Rev. Immunol. 2018, DOI: 10.1038/nri.2018.3). Compared with healthy cells, cancer cells sport a higher density of glycan structures terminating in sialic acid, the ligand for Siglecs. Could it be that Siglecs act as additional immune-cell brakes that a drug could release?
Biochemically, Siglecs that dampen immune-cell activity use the same inhibitory pathway that PD-1 uses. Bertozzi reasoned that targeting these Siglecs or their sialic acid ligands could yield new immune therapies to expand the therapeutic reach of the current batch.
In 2013 her lab published a paper that suggested her hunch was correct. The study involved inserting synthetic sialic acids, at varying quantities, into the membranes of tumor cells. The sugars created a blockade, protecting the tumor cells from attack by natural killer (NK) cells (Nat. Chem. Biol. 2013, DOI: 10.1038/nchembio.1388). These lab-dish experiments were “a direct demonstration of cause and effect,” says Bertozzi, who led the work. “You could cause immune suppression by putting extra sialic acids on tumor cells.”
The concept supported earlier cell culture experiments by Crocker and reinforced research coming out in 2014 from Varki’s lab and a team led by Stephan von Gunten at the University of Bern. Von Gunten analyzed mouse models and human tumor samples to confirm that interactions between Siglecs and sialic acids turn down tumor-killing activity in immune cells.
Since Siglecs populate not only T cells but also first responders such as macrophages and NK cells, creating immunotherapies that target Siglec–sialic acid interactions could in theory unleash a more powerful immune response than current checkpoint blockers.
After talking with Bertozzi, “I basically had a vision for what Palleon was going to be,” says Broderick, who invited her and Crocker to join Palleon as cofounders. “All the other things I was working on, basically I dropped that day and said, You know what, this is a huge cancer opportunity in a whole field that people aren’t in.”
The conversation stunned Bertozzi, too. Many of her biologist and physician collaborators are daunted by the complexity of glycans. Broderick, however, was unfazed. “He was not handicapped by the fear and bias against glycobiology as an area that’s ripe for biotech,” she says.
Another cloak
Searching for selectivity
But Broderick knew that devising a glycoimmune drug strategy wouldn’t be easy. Siglecs are complex. Researchers have identified 14 of them in humans and 9 in mice—and each one recognizes a particular set of sialic acid–containing structures. Although many cancers likely have Siglecs that can be blocked by a drug, some tumors might express, say, Siglec-3, Siglec-7, and Siglec-9, while others could display an entirely different combination. “Precisely which Siglecs are most important is going to be tumor specific, and maybe even individual specific,” Bertozzi says. “How many Siglecs do you need to block?”
Targeting the glycans that Siglecs bind presents a different challenge. Unlike most protein receptors, which recognize a single ligand, each Siglec links to a multitude of glycan-containing structures.
“Oh my gosh, this is such a complicated interaction. How are you going to block this?” Peng recalls thinking in the summer of 2016, while deciding whether to leave MedImmune’s stability and security for the adventure of a start-up.
She soon learned that Bertozzi had a workaround: direct an enzyme to tumor cells to cut off sialic acids. No matter how numerous or complicated, all Siglec ligands contain terminal sialic acids, Bertozzi reasoned.
To hit cancer cells but not normal ones, her team linked the sialic acid–trimming enzyme to an antibody specific for a common tumor antigen—in this case, the antigen HER2, which is found on many breast cancers and other solid tumors. In lab-dish experiments, when the enzyme-antibody conjugate stripped off sialic acids, it exposed tumor cells to killing by NK cells (Proc. Natl. Acad. Sci. U.S.A. 2016, DOI: 10.1073/pnas.1608069113).
“I think that is a nice approach,” says Michael O’Dwyer, who studies the role of glycans in blood cancers at the National University of Ireland. “You’re getting rid of all the sialic acid that’s there and, in doing so, eliminating any Siglec ligands that might be present on the cell surface.” The strategy is Siglec agnostic.
Peng joined Palleon in the fall of 2016 hoping to turn this concept into a viable therapeutic.
Several of the company’s investors, however, balked at the idea. They were fine investing in a strategy to block Siglecs, since that could be achieved with neutralizing antibodies, conventional biologics with a clear development path. However, “the idea of a therapeutic enzyme was just too off the wall,” Broderick recalls.
But Peng was determined. Siglec–sialic acid interactions are “an important, hard-wired immunosuppression mechanism that tumors take advantage of,” she told Broderick. “If you want to [target] glycoimmune checkpoints, you’ve got to own both pieces—receptor and ligand.”
Broderick agreed to give the sialidase a shot. He proposed shifting a small fraction of the company’s budget to this side project. The investors acquiesced, though one privately told the CEO he’d “need a team of 30 to make this thing.” Peng had 1.5 full-time employees.
Within a year her team did two key things. First, they converted Bertozzi’s sialidase-antibody molecule from a chemical conjugate to a genetic fusion, making the therapeutic easier to manufacture. Second, they showed it slowed tumor growth in several mouse models. For the animal studies, they teamed up with Heinz Läubli, a University of Basel physician-researcher who serves on Palleon’s scientific advisory board. In Läubli’s mouse model, the sialidase-HER2 antibody fusion cured three of eight mice. When the drug was given in combination with current checkpoint blockers (anti-PD-1 or anti-PD-L1 antibodies), all the mice were cured. After seeing that data, “I stayed up almost half the night,” Peng says. “I was very excited.”
A larger chunk of the company’s funds were shifted toward the sialidase project. Combining rational design and directed evolution approaches, the team modified the enzyme to make it stabler and safer in humans.
Gaining traction
While Palleon was making progress targeting Siglecs’ ligands, others in the field focused on directly inhibiting the membrane receptors—for example, Siglec-9, which binds a wider range of glycan structures than other Siglecs do. Recent work shows that Siglec-9 shifts macrophages toward tumor-promoting behaviors and gets overexpressed on tumor-associated T cells.
At the 2018 American Association for Cancer Research (AACR) meeting, Innate Pharma reported that its Siglec-9 antibodies increase NK cells’ tumor-killing activity in lab-dish experiments. Alector, a biotech company harnessing Siglecs to fight neurodegenerative diseases, has jumped on the immuno-oncology bandwagon, pursuing numerous targets, including Siglec-5, Siglec-7, and Siglec-9. “We have indications of preclinical activity but are not disclosing details,” CEO Arnon Rosenthal wrote in an email.
Signaling growing interest in a neglected area, this year’s AACR meeting featured the association’s first member-initiated glycoscience session. The symposium, “Regulation of the Immune Microenvironment by Glycosylation,” was chaired by Joseph Contessa, a radiation oncologist at Yale University whose team hopes to commercialize a molecule it identified as a partial inhibitor of glycosylation (Cell Chem. Biol. 2018, DOI: 10.1016/j.chembiol.2018.07.005). Since tumors depend on glycoproteins to survive and progress, Contessa says, drugs that keep sugars from tacking onto proteins could fight cancer broadly, much as chemotherapy or proteasome inhibitors do.
Advertisement
Just before the meeting, Yale immunologist Lieping Chen, who did fundamental work that led to PD-1 and PD-L1 immunotherapies, published a paper identifying Siglec-15 as an immune suppressor (Nat. Med. 2019, DOI: 10.1038/s41591-019-0374-x). His team found Siglec-15 in a screen for proteins that inhibit T-cell activation. In healthy cells, Siglec-15 is found at low levels on the surface of macrophages. But in cancer, Siglec-15 expression surges—not only on macrophages but also on tumor cells.
Chen also found that cancer cells with high levels of Siglec-15 generally do not express the PD-L1 inhibitory ligand, suggesting that these mechanisms that tumors use to evade immune detection don’t overlap. The finding opens the possibility that people who don’t respond to the current checkpoint inhibitors could benefit from antibodies that block Siglec-15, says Sol Langermann, chief scientific officer of NextCure, a biotech company founded by Chen that is developing Siglec-15 antibodies to treat cancer. Last fall the company began enrolling patients in an early-stage trial of its experimental treatment for advanced solid tumors.
Palleon, meanwhile, has now committed the majority of its cash to the sialidase-antibody program and hopes to start clinical trials of its lead drug candidate by early 2021. Despite preclinical success, uncertainties loom. For one, trimming sialic acids exposes sugars that could serve as ligands for other receptors. “We don’t understand completely what we are doing at a mechanistic level,” Läubli says.
Contessa is excited that Siglecs are gaining traction. “It’s always great in science when you get multiple independent laboratories coming up with, maybe not identical solutions, but similar ones. And then multiple companies sprouting up,” he says. “I think there’s something there.”
Esther Landhuis is a freelance journalist based in the San Francisco Bay Area.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter