Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Cage snatches chloride with C–H bonds
Rigid cryptand structure has record-breaking affinity for the anion
by Bethany Halford
May 30, 2019
| A version of this story appeared in
Volume 97, Issue 22

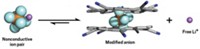
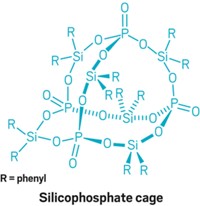
A cage-like cryptand molecule selectively captures chloride ions and binds them with record-setting strength. The molecular cage’s chloride-sequestering power comes entirely from C–H bonds, which have long been considered weak hydrogen-bond donors (Science 2019, DOI: 10.1126/science.aaw5145). Besides offering proof of the power of C–H bonds to bind anions, the cage molecule could be used to prevent chloride-based corrosion, say the Indiana University Bloomington chemists who created it.
The cage’s ability to snatch chloride ions is superior to all other known chloride-binding molecules, says Amar H. Flood, who led the research team. Its affinity for chloride is so strong that the team was never able to isolate the cage without chloride ions bound. It even pulled chloride from silica gel during chromatographic separation. The chemists spent a year trying to remove all traces of chloride from samples of the molecule but were unable to purge the last 10%. The cage is also extremely selective for chloride, nabbing the ion instead of several others.
Inspiration to build the cage dates back to 2012 when Yun Liu, the study’s lead author, was challenged by his wife, Jie Fu, also a chemist, to create a cage similar to one she’d seen from Kristin Bowman-James’s group at the University of Kansas. Fu suggested swapping out the amides in that anion-grabbing cage for triazoles, which added rigidity.
“I never thought that rigidity could deliver so much strength to the binding and have such a big effect on selectivity,” Flood says.
The new molecule “changes the way we must think about anion recognition,” says Jonathan L. Sessler, a supramolecular chemistry expert at the University of Texas at Austin. “This work adds a new dimension to the timely topics of anion recognition, extraction, and separation.”
“These results are especially of note because they indicate that very strong, selective anion binding can be achieved even using nontraditional hydrogen-bond C–H group donors with some assistance from the cryptand cage,” Bowman-James adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter