Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Novel P and N chemistry
2 groups made new molecules from the unstable PN combination
by Sam Lemonick
September 26, 2021
| A version of this story appeared in
Volume 99, Issue 35
Researchers have put phosphorus and nitrogen to new use. One group made a prismatic P3N3 molecule for the first time (Nat. Commun. 2021, DOI: 10.1038/s41467-021-25775-1), and a second group found a molecule that can add phosphorus mononitride to another molecule under milder reaction conditions than was previously possible (ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-zxtmf). P and N are an unstable and often explosive combination in molecules, something both teams were able to overcome, at least for a short while.
At the University of Hawaii at Manoa, astrochemist Ralf Kaiser and colleagues were ionizing mixtures of phosphine and dinitrogen ices in an attempt to simulate interstellar space chemistry. They didn’t find what they were looking for, Kaiser says, but they noticed in their mass spectrometry data a signal for prismatic P3N3. The molecule had never been synthesized before. It shares its shape and valence electron structure with prismane, once proposed to be the structure of benzene. Scientists have synthesized just a few molecules with this geometry, all as cores of larger organometallic complexes; the strain of repulsive interactions between nonmetal elements makes this geometry very unstable. The group’s prismatic P3N3 survived at least 10 µs. Kaiser hopes the discovery may help scientists find ways to produce longer-lived versions of it and other isomers. He thinks it may lead to new energetic molecules like explosives, while Massachusetts Institute of Technology inorganic chemist Christopher (Kit) Cummins writes in an email, “Metastable compounds such as this one may offer synthetic pathways to novel classes of electronic materials.”
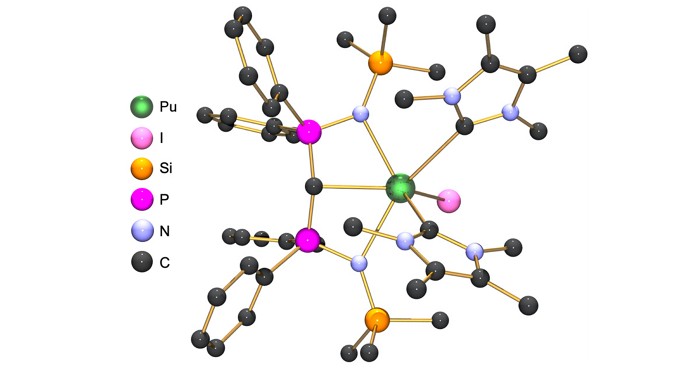
Cummins led the group that reported the phosphorus mononitride work, which has not yet been peer-reviewed. Cummins and collaborators set out to develop a method to add phosphorus mononitride—a molecule seen only in space and high-temperature pyrolysis reactions—to a transition-metal complex. They made a molecule that explosively decomposes to anthracene, N2, and PN, which they then used to make an iron and PN complex.
The two projects share one scientist: André K. Eckhardt, who worked with Kaiser’s group as a PhD student and is now a postdoctoral researcher with Cummins and at Harvard University. Eckhardt says Cummins’s group is exploring building larger P and N molecules.
CORRECTION
This story was updated on Sept. 28, 2021, to say that the prismatic P3N3 survived at least 10 µs, not about 10 µs.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter