Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Theoretical Chemistry
Model explains stability of extreme-pressure helium compounds
Study deepens understanding of noble-gas reactivity, inertness, and geochemistry
by Mitch Jacoby
April 2, 2018
| A version of this story appeared in
Volume 96, Issue 14
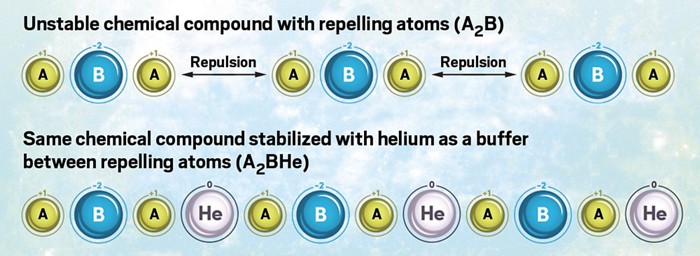
Helium is best known as the lightweight gas that keeps balloons aloft. Numerous technologies also rely on the element, for example, as a refrigerant and gas that helps enclosed atmospheres remain inert. Now, a study shows helium can play a role as peacekeeper between bickering atoms (Nat. Commun. 2018, DOI: 10.1038/s41467-018-03284-y). An international team of researchers led by Maosheng Miao of California State University, Northridge, and the Beijing Computational Science Research Center has developed a theoretical model that explains how some helium compounds that would otherwise be unstable under tremendous pressures—such as in the interiors of planets—can remain stable. The model (shown) explains that in ionic compounds with unequal numbers of cations (A) and anions (B)—for example, ones with A2B stoichiometry—extreme pressures drive like-charged ions together, causing repulsion and instability. At those pressures, helium can take up a position between repelling ions and alter long-range coulombic interactions, thereby stabilizing the compounds. The study helps explain the recent surprising observation of stable high-pressure helium-sodium compounds and predicts that helium may be found in other compounds in Earth’s lower mantle.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter