Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Building bridges out of azaarenes
Photochemical reaction spurs nitrogen-containing aromatic compounds to sacrifice stability and form bridged molecules
by Bethany Halford
March 26, 2021
| A version of this story appeared in
Volume 99, Issue 11

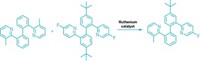
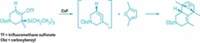
Organic chemists tend to swoon at the sight of [4 + 2] cycloadditions, a chemical dance in which a diene and an alkene join via two new bonds to create a six-membered ring. While many dienes are willing to partner with alkenes this way, one source of dienes—aromatic molecules—has been reluctant to engage in such behavior. That’s largely because getting these molecules to give up their aromatic stability requires quite a reactive kick. Now, chemists have coaxed azaarenes, including quinolines, isoquinolines, and quinazolines, to break out of their aromatic ruts and form bridged bicyclic molecules by reacting with alkenes (Science 2021, DOI: 10.1126/science.abg0720). A team led by M. Kevin Brown of Indiana University Bloomington; Kendall N. Houk of the University of California, Los Angeles; and Frank Glorius of the University of Münster reports the transformation, which uses an iridium photosensitizer, blue light, and an acid (example shown). The regio- and diastereoselective reaction forms bridged bicyclic molecules that require multiple steps to make by other methods. The reaction could be useful to medicinal chemists making unique and complex chemical scaffolds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter