Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists make arrays of click chemistry–ready azides efficiently and safely
Reaction converts primary amines to azides to make large libraries of compounds
by Bethany Halford
October 2, 2019
| A version of this story appeared in
Volume 97, Issue 39

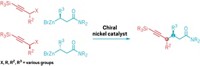
A new reaction that makes azides from virtually any primary amine gives chemists the ability to create vast libraries of compounds poised for click chemistry. The transformation, discovered by Jiajia Dong of the Shanghai Institute of Organic Chemistry, K. Barry Sharpless of Scripps Research in California, and colleagues, will have applications in many fields, including organic synthesis, medicinal chemistry, chemical biology, and materials science.
Click chemistry—in which two highly reactive compounds assemble in a reliable manner without any by-products—has been a valuable tool in many fields. The most popular of the click reactions, the copper(I)-catalyzed azide-alkyne cycloaddition, or CuAAC, is commonly used by chemical biologists, materials scientists, and polymer chemists. CuAAC weds an azide and an alkyne to make a triazole that connects two different molecular entities.
As useful as CuAAC has been for scientists, synthesizing the azide is not trivial. Triflyl azide, a popular reagent for making azides from primary amines, takes a long time to react even with the help of a metal catalyst and is toxic. Further, both triflyl azide and another popular reagent, imidazole-1-sulfonyl azide hydrochloride, pose an explosion risk. Dong, Sharpless, and colleagues reasoned that any reagent that would be good at making azides would also have to be highly reactive, which would make it prone to explosion when stored.
While working on an entirely different reaction, the researchers discovered that fluorosulfuryl azide could transform primary amines into azides in a matter of minutes—a property they realized could help them create compounds for click chemistry.
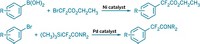
Fluorosulfuryl azide doesn’t need to be purified or stored because the chemists generate it in the reaction flask directly from an imidazolium fluorosulfuryl triflate salt and sodium azide. That way, they sidestep any risks of explosion. What’s more, toxicity tests on rodents show that the starting triflate salt poses minimal risk of toxicity (Nature 2019, DOI: 10.1038/s41586-019-1589-1).
“With the introduction of click chemistry, in particular CuAAC, azides have become an extremely valuable functional group,” says Valentin Wittmann, a chemistry professor at the University of Konstanz who has studied methods for making azides. The report by Dong and Sharpless, he says, represents a major step toward preparing azides safely and efficiently.
“It looks to be a simple and reliable route to a diverse array of azides,” says Ethan Goddard-Borger, a chemical biologist at the Walter and Eliza Hall Institute of Medical Research. “Only time and the collective experience of the chemistry community will reveal how useful the new method is. My lab will certainly be giving it a try.”
Dong says the new reaction makes it possible to create libraries of azides composed of 1,200 compounds. He hopes the reaction will speed the discovery of useful new molecules, particularly pharmaceuticals, using click chemistry. That vision is already taking shape, says Mike Petrassi, vice president of medicinal chemistry at Calibr, a biomedical research institute. He says triazole libraries made via Dong and Sharpless’s method have already yielded a promising compound for fighting tuberculosis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter