Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enzyme and copper combo builds amide bonds
One-pot reaction offers a greener route to this important molecular motif
by Bethany Halford
January 27, 2022
| A version of this story appeared in
Volume 100, Issue 4

Even though amide bonds are everywhere—they hold proteins together and are essential components in many drugs—forging this molecular motif in a reaction flask can be challenging. For example, making amides by coupling amines and carboxylic acids may require expensive reagents and generates organic solvent waste.
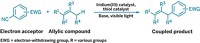
Looking for an environmentally friendly alternative, chemists at the University of Manchester led by Jason Micklefield found that they could make amides by combining enzyme and copper catalysts (Nat. Commun. 2022, DOI: 10.1038/s41467-022-28005-4). Their method uses nitrile hydratase enzymes to transform aryl and alkyl nitriles into a primary amide intermediate. A catalyst, made from inexpensive, abundant copper, then tacks an aryl group onto the amide’s nitrogen. They used the reaction to create more than 50 amides, including a sodium channel inhibitor (shown).
The transformation takes place in a single reaction vessel using an environmentally benign aqueous buffer and a 2-propanol solvent system. The chemists found the reaction works best if they don’t purify the nitrile hydratase enzymes and instead add Escherichia coli cells containing the enzymes to the reaction flask. They speculate that this is because the cell membrane prevents the enzymes from interacting with the copper catalyst. They also discovered that adding surfactant to the reaction mixture boosted yields, particularly with aliphatic nitriles. This is probably because the surfactant creates organic microcompartments where the primary amide intermediate, iodoarene coupling partner, and copper catalyst can interact.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter