Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Light and acid clip carbon out of azaarenes
Reaction transforms quinoline N-oxides into N-acylindoles, giving chemists a way to modify a complex molecule’s core
by Bethany Halford
May 3, 2022
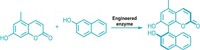
Chemists can now quickly downsize certain azaarenes from six-membered rings to five-membered rings in a reaction that’s driven by light and acid. The transformation, which turns quinoline N-oxides into N-acylindoles (example shown), offers a way to remodel a complex molecule’s core without changing its substituents. Such reactions provide a simple way for medicinal chemists to make an array of molecule derivatives when looking for drug candidates.

The reaction was developed by chemists in Mark D. Levin’s group at the University of Chicago and at Merck & Co. Last year, Levin reported a reaction that expanded indoles by one carbon to make quinolines. When graduate student Jisoo Woo was looking for a project, Levin suggested trying the reverse—contracting heterocycles by one carbon. “I wasn’t 100% sure of how to even tackle it,” Levin says. Woo found publications that used photochemistry with a mercury lamp to turn quinoline N-oxides into N-acylindoles, but the reaction only worked on certain starting materials. Woo was able to develop the reaction to make it generally applicable (Science 2022, DOI: 10.1126/science.abo4282).

“We had to really reengineer the chemistry to solve the problems with it,” Levin says. One key insight was to switch from using the mercury lamp, which put out multiple wavelengths of light, to a 390 nm light-emitting diode. This change made the reaction much cleaner because it only initiated a single photochemical reaction.
Chemists can easily replace the core structure of a potential drug candidate in computer software through a process called “scaffold hopping,” says Jürgen Bajorath, a University of Bonn professor who studies such modifications. While it’s easy to change core structures with a computer, such scaffold hops can be difficult to implement synthetically. “The photochemical approach reported by Levin and colleagues enables carbon atom deletions in aromatic heterocycles and the generation of new scaffolds in easier ways than has been possible so far,” Bajorath says in an email.
Next, Levin hopes to find reactions that clip other carbons out from the quinoline core.
CORRECTION:
The story was updated on May 5, 2022, to correct the name of the graduate student in the caption. It is Jisoo Woo, not Jisoo Wool.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter