Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Radical new way to control chirality
Two-catalyst radical reaction adds a variety of groups to nitrogen rings enantioselectively
by Stu Borman
April 5, 2018
| A version of this story appeared in
Volume 96, Issue 15

Nitrogen rings with attached chiral organic groups are common motifs in pharmaceuticals, agricultural chemicals, and bioactive small molecules. Medicinal chemists frequently seek to add chemical groups with specific chirality to nitrogen heterocycles, but their toolbox is limited.
Reactions like catalytic asymmetric reduction can control enantioselectivity in nitrogen heterocycle additions, but typically require preinstalling reactive groups on the rings and multiple steps. An alternative is a free-radical addition called a Minisci-type reaction, but it is not enantioselective and often adds groups to several ring positions on the heterocycle.
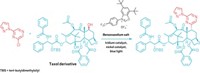
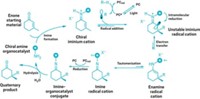
Robert J. Phipps and grad students Rupert S. J. Proctor and Holly J. Davis at the University of Cambridge now propose a fix for those drawbacks. They developed a Minisci-type reaction that uses two catalysts to add N-acyl α-amino alkyl radicals to nitrogen heteroarenes (Science 2018, DOI: 10.1126/science.aar6376). The approach creates a chiral center enantioselectively on a carbon attached to the ring, with tight control over the ring position of the addition and in fewer steps than catalytic asymmetric reduction.
The α-amino radicals the reaction adds to nitrogen rings can contain a broad range of other organic groups, including amines, thioethers, and aryl iodides. The products “possess structural features highly desirable in pharmaceutical compounds: a basic heteroarene, protected primary amine, and a defined stereocenter,” all in proximity, Phipps and coworkers note.
The team shines blue light-emitting diodes at the reaction mixture to trigger the reaction. A chiral acid catalyst activates the heterocycle, and an iridium photocatalyst mediates electron transfers required to generate the initial radical and to form the final product.
“This is an amazing paper,” says Varinder K. Aggarwal of the University of Bristol. The group “has uncovered a very simple, although mechanistically complex, method to combine readily available amino acids and heterocycles in a Minisci-type reaction with very high levels of enantiocontrol. The simplicity of the procedure, the ready availability of the reaction partners, and the high value of the products makes this chemistry highly attractive.”
“This is a really creative contribution from a young scientist who is new to the synthetic organic academic world,” adds David MacMillan of Princeton University, speaking of Phipps. “The quality and insight speak volumes for his potential, and I cannot wait to see what comes next from his lab. This is a significant advance that allows transformations generally viewed as being racemic by their inherent nature to be readily translated into enantioselective processes. This will definitely be used by medicinal chemists when they need to make reasonable amounts of enantioenriched intermediates, and it should be amenable to scale-up and manufacturing.”
At present, the group is not taking steps to commercialize the chemistry. “We are keen just to get the results out there for people to use,” Phipps says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter