Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Reaction couples aromatic molecules with phosphorus rather than metal
Strategy assembles complex, druglike molecules quickly
by Bethany Halford
November 16, 2018
| A version of this story appeared in
Volume 96, Issue 46

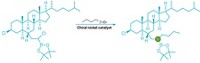
To forge carbon-carbon bonds between two aryl groups, chemists typically turn to metal-catalyzed cross-couplings—powerful transformations that garnered their inventors the 2010 Nobel Prize in Chemistry. But these reactions can be problematic when applied to nitrogen-containing aromatic compounds. The reactants can be tough to prepare and can also poison the metal catalysts. Chemists at Colorado State University have developed an alternative coupling for nitrogen-containing aryl groups—specifically, pyridines and diazines—that eschews metal for phosphorus.
The reaction works by assembling two nitrogen-containing aryl groups onto a phosphorus atom as ligands. Acidic alcohol triggers C–C bond formation between the two ligands (Science 2018, DOI: 10.1126/science.aas8961).
“These ligand-coupling processes had been observed before, but no one had used them practically,” notes Andrew McNally, who led the research effort with colleague Robert S. Paton. The key, McNally says, was designing a good phosphorus reagent.
The reaction takes place in stages, Paton notes, unlike metal-catalyzed coupling reactions, in which the steps take place all at once. This, he says, “allows you to think about how you might control this reaction and also, more excitingly, how it can be explored to make other types of C–C bonds.”
McNally says the reaction will help medicinal chemists construct complicated drug candidates quickly. “You can take small, relatively complex fragments that would be in a pharmaceutical company’s collection and very rapidly couple those together.” He acknowledges that the phosphorus by-products aren’t environmentally friendly but says they are less problematic on the small scale of drug discovery.
David Rees, chief scientific officer at Astex Pharmaceuticals, says the reaction is attractive to the pharmaceutical industry. “Although it has not been used to make a known drug, the authors show the methodology works for druglike substrates containing polar functional groups, and they’ve applied it to make new heterobiaryl derivatives of drugs that would be difficult to access with existing methods,” he says.
Alexander Radosevich, an organic chemist at Massachusetts Institute of Technology, adds that the research “reshapes an esoteric transformation in main-group chemistry into a powerful synthetic sequence.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter