Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biocatalysis
Engineered Diels-Alder enzyme is fast and selective
Design and directed evolution combine to make an artificial enzyme with record-breaking performance
by Leigh Krietsch Boerner
February 1, 2021

Using computational design and directed evolution, scientists have successfully created a metalloprotein that can catalyze a commonly used organic reaction more efficiently than any known enzyme (Nat. Chem., 2021 DOI: 10.1038/s41557-020-00628-4). A team led by Donald Hilvert of ETH Zürich and Gonzalo Jiménez-Osés of Basque Research and Technology Alliance engineered a new enzyme to catalyze the Diels-Alder reaction, which breaks two double bonds to form a new cyclic compound and which is widely used in industrial and laboratory synthesis. The group’s protein produces their target compound more than an order of magnitude faster than any characterized Diels-Alderases and with >99% selectivity.
Often catalyzed by a Lewis acid metal, Diels-Alder reactions can make multiple products from the same set of reactants, especially if those reactants contain multiple double bonds. Enzymes can favor particular products by locking the reactants and the metal catalyst in a specific orientation relative to each other. But past enzymatic Diels-Alder reactions tend to take days to create their products and only have moderate to good regioselectivity. That is, the enzyme targets a specific set of bonds in the molecule, but these bonds can still break and form in various ways, producing more than one product.

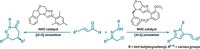
To make a better enzyme, the team first used density functional theory to examine the binding pocket of a 97-amino-acid-long zinc-binding protein called MID1sc, hoping to capitalize on the zinc at its core to catalyze the Diels-Alder reaction. They identified certain sites to mutate as targets that the models predicted might lower the reaction barrier to making a Diels-Alder product with high regioselectivity. The researchers put the enzyme through seven rounds of directed evolution—a method that picks out variants with improved function from a library of mutated protein candidates—looking for new proteins with improved Diels-Alder activity.
The resulting protein was over one order of magnitude faster and more specific at producing a particular isomer and compound, in this case, the endo dihydropyran.
The key to both the high activity and regioselectivity of this enzyme was the fit of the transition state into the binding pocket, Hilvert says. During the course of evolution, this pocket’s shape shifted, bringing the substrates together at a better angle for them to react, creating a stabilizing web of hydrogen bonds, and pulling off the amine H from 3-vinylindole so it can attack the heterodiene more efficiently (shown). “This was not planned, this came out of the [directed] evolution,” Hilvert says.
In the computational studies, the researchers showed that the unwanted enantiomer would not fit into the binding pocket, while the correct enantiomer for the observed products “fits beautifully, like a hand in a glove,” he says. “We were able to take a scaffold that had zero starting activity and turn it into something was really quite active,” Hilvert says. “It’s a very big boost in efficiency.”
The the team targeted the reaction of azachalcone with 3-vinylindole because the products contain nitrogen motifs commonly found in pharmaceutical compounds. This work is an important step towards being able to design artificial enzymes to make important organic compounds with high stereo- and regioselectivity, says William F. DeGrado, pharmacological chemist at the University of California, San Francisco.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter