Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
C-H Activation
Ligand spiral activates elusive γ C-H bond
Method selects this bond over more reactive β C-H
by Leigh Krietsch Boerner
May 27, 2022

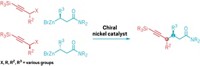
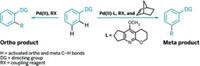
To find the shortest and easiest path to a target drug compound, chemists want to be able to control every bond. Now another carbon-hydrogen bond is within their grasp. Jin-Quan Yu and team at Scripps Research in California have figured out how to get a catalyst to specifically target the γ C-H of a dicarboxylic acid for a cyclization reaction, skipping over the more reactive β carbon in the process (Science 2022, DOI: 10.1126/science.abq3048).
The group used adipic and pimelic acids as a starting material, widely available industrial commodity chemicals. Yu and coworkers took a palladium catalyst and a specially designed quinoline-pyridone ligand to synthesize an array of butyrolactones (example shown), common cyclic motifs in drug compounds. The hard part of this method was activating a γ C-H on the acids because there was a β C-H right next door, Yu says. “But it turns out by matching the design of the ligand, we could favor γ in the presence of β,” he says. The trick was to create a stable intermediate with the Pd and two rings from the ligand, which formed a kind of spiral, Yu says. When the intermediate makes two six-membered rings, the γ C-H lines up perfectly with the target atoms at the other end of the molecule to form the desired new bond and complete the cyclization. Yu and team used the method to make complex natural products, including the anticancer compound myrotheciumone A.
Traditional routes to make butyrolactones are long, so this synthetic shortcut is exciting, says M. Christina White, organic chemist at the University of Illinois Urbana-Champaign. Although the method requires starting materials with two carboxlyic acids in the right location, chemists can later swap out the unreacted carboxylic acid for another functional group.
The new method adds a long-missing tool to the C-H activation toolbox, Yu says.
α C-H activation is well established, but the products of β and γ activation make up a large percentage of drug compounds, he says. This method delivers the γ compounds.
CORRECTION:
This story was updated on June 18, 2022, to correct the starting materials in the reactions. The team used adipic and pimelic acids, not just adipic acids.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter