Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Attached to MOFs, frustrated Lewis pair catalysts become recyclable
Tunable pairing opens new avenues for heterogeneous catalysis
by Tien Nguyen
September 27, 2018
| A version of this story appeared in
Volume 96, Issue 39
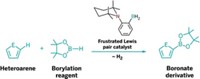
Frustrated Lewis pairs (FLPs) stepped into the synthetic limelight just over a decade ago. Composed of a Lewis acid and a Lewis base whose blocked inclination to bond grants the molecules great powers, the main-group molecules were hailed for catalyzing hydrogenation reactions previously accomplished only with transition metals. The use of FLPs has been limited, however, by their lack of recyclability and their sensitivity to air and moisture.
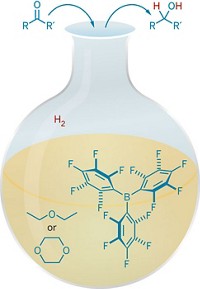
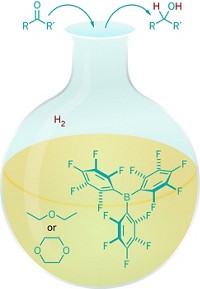
Now, researchers led by Shengqian Ma at the University of South Florida have developed stable FLPs. Anchored inside a metal-organic framework (MOF), they efficiently catalyze hydrogenation and imine reduction reactions (Chem 2018, DOI: 10.1016/j.chempr.2018.08.018). With simple filtration, the catalyst can be recycled in the latter reaction at least seven times without loss of activity.
To construct the MOF-FLP pair, the team docked an amine Lewis base at the MOF’s open metal sites and then attached a boron Lewis acid (shown). The team then compared the heterogeneous MOF-FLP’s ability to catalyze reactions with that of a homogeneous catalyst. While the researchers observed comparable yields for the hydrogenation reaction, Ma says the MOF-FLP showed “interesting” steric and size selectivity in the imine reduction. The MOF-FLP matched the homogeneous catalyst’s high yield unless the substrate contained a buried imine, suggesting that the MOF environment restricts access to certain substrates. Substrates beyond a certain size were not reduced, likely because they can’t squeeze through the MOF’s pores, the authors say.

“The beauty of MOFs is that they’re very tunable,” Ma says. Future MOF designs could easily introduce chirality or superhydrophobicity to expand the FLP catalysts’ reaction scope and durability, he says.
The University of Toronto’s Douglas W. Stephan, whose group pioneered the use of FLPs, calls the work a “remarkably clever and facile strategy.” The readily handled MOF-LPs avoid the air and moisture sensitivity of most homogeneous FLP catalysts, and their recyclability could also lower costs, Stephan says. The study, he adds, “foreshadows a vast potential for an array of new stable, recyclable, and selective FLP catalysts embedded in MOFs.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter