Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Free-floating electrocatalysts outperform tethered ones
Detaching particles from the electrode surface frees them from electrical fatigue
by Mitch Jacoby
February 27, 2020

Different isn’t always better. But a new study shows how radically redesigning a chemistry workhorse can have huge benefits. By letting their catalytic particles float freely in solution, untethered from the electrodes typically used to run electrocatalytic reactions, researchers based in China and the US boosted the particles’ catalytic activity by orders of magnitude and extended their lifetimes (CCS Chem. 2020, DOI: 10.31635/ccschem.020.201900065). The findings suggest ways to improve catalyst performance and may advance efforts to make industrial synthesis greener through wider use of electrochemistry.
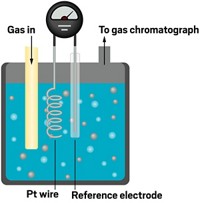
Electrocatalysts drive the chemistry that underpins numerous clean energy devices, including electricity-generating fuel cells and hydrogen-producing water splitters. To evaluate new electrocatalysts, researchers typically bind the materials to an electrode, immerse it in electrolyte solution, power up the electrode, and monitor the ensuing chemistry. In many cases, the catalytic electrode begins working well, then quickly starts to fail.
Several unwanted processes conspire to degrade catalyst performance. Yi-Ge Zhou and Yijin Kang of the University of Electronic Science and Technology of China, and Jiaxing Huang of Northwestern University hypothesized that the damage is exacerbated because the electrode is always “on,” and the constant current fatigues the catalyst particles.
The team proposed that various factors that cause catalytic activity to wane—particle agglomeration, poisoning by reaction intermediates, detachment from the electrode, and others—could be mitigated by minimizing the particles’ exposure to electric current. If the particles were left free-floating in solution, they would experience the voltage effects only while they briefly contact the electrode, which is the moment when they catalyze reactions.
To test the idea, the team set up cells using a common reference catalyst—platinum nanoparticles on carbon—and studied three common reactions known for performance decay: oxygen and hydrogen evolution and methanol oxidation. Compared with immobilized catalysts, in all cases, the fluidized catalysts provided higher per-particle catalytic activity—in one case, nearly 1000 times as high, and they showed greater resistance to fatigue and degradation. Microscopy analysis showed that after just a few minutes of reaction time, immobilized particles had already begun degrading and changing morphology. In contrast, fluidized particles showed little change even after 10,000 minutes.
“The work is groundbreaking in that it takes particle-impact experiments from the academic study of single nanoparticle electrocatalysis and suggests that they can be scaled up with considerable benefit,” says University of Oxford’s Richard G. Compton. Compton is referring to single-particle methods for studying reaction mechanisms, analytical techniques developed by his group and that of Allen J. Bard of the University of Texas, Austin. The approach has potential for broad application in electrocatalytic systems, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter