Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Reversing rules of arene chemistry
New norbornene compound overcomes ring substitution limitation
by Tien Nguyen
July 1, 2018
| A version of this story appeared in
Volume 96, Issue 27
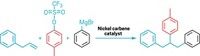
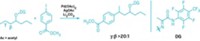
Electronics reign in the realm of electrophilic aromatic substitutions. In these reactions, six-membered rings with electron-rich substituents point reactants to adjacent or opposing positions on the ring, while compounds with electron-poor groups place them on the carbon in between. Medicinal chemists have long sought methods to free themselves from these conventions. Reactions using a palladium catalyst combined with a norbornene cofactor offer reversed site selectivity in aryl iodides, but they come with caveats. Guangbin Dong’s lab at the University of Chicago has developed a novel norbornene compound (shown) that lifts one of those limitations (Nat. Chem. 2018, DOI: 10.1038/s41557-018-0074-z). Called the ortho constraint, a substituent had to occupy the ortho—in other words, adjacent—position on the ring next to iodine to avoid overreaction or norbornene getting stuck on the ring. The researchers were able to forgo this substituent by incorporating a floppy alkyl chain at the bridgehead position of norbornene, which they suggest disfavors the side reactions. Dong and coworkers demonstrated the reaction’s complementary site selectivity by adding amine, acyl, and aryl electrophiles in tandem with various nucleophiles, including hydride, to arene substrates possessing a variety of electron-donating and electron-withdrawing groups. In one example, the researchers accomplished a late-stage amination of the pesticide strychnine that had previously taken seven steps in only two steps.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter