Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Fluorination
Chemists fracture rings to install fluorine
Reaction opens unstrained nitrogen rings furnishing alkyl fluorine compounds
by Tien Nguyen
July 14, 2018
| A version of this story appeared in
Volume 96, Issue 29

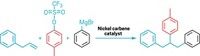
To construct new bonds, as chemists are wont to do, they must first break bonds. But some bonds give way easier than others. Researchers can readily split carbon-carbon double bonds for further functionalization, yet they struggle to crack relatively inert, C–C single bonds. A new method developed by Richmond Sarpong and coworkers at the University of California, Berkeley, now cleaves C–C bonds in nitrogen-containing rings (Science 2018, DOI: 10.1126/science.aat6365). Using an excess of a silver salt and a common fluorinating reagent, the reaction (example shown) unfurls the ring to a linear alkyl chain with a fluorine atom at one end, a functional group coveted by chemists for its ability to modulate a molecule’s properties. In some cases, the method also introduces a formyl group on the nitrogen at the other end. The team proposes that the reaction proceeds through oxidation followed by radical ring opening. They successfully opened four-membered rings and larger ones, but five-membered rings were mostly just oxygenated. The researchers also demonstrated the ring-opening fluorination of two peptides, a strategy that could be immediately applicable in drug discovery or biological uses, Sarpong says. In addition, the team showed the geminal difluorination of rings using 0.25 equivalents of a silver salt and hope to lower the amount of metal needed in future reactions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter