Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Fluorination
Trifluoromethylated heteroatoms go with the flow
Making reactive fluorinated anions on demand may reduce wasteful and hard-to-scale reagents
by Brianna Barbu
September 5, 2024
| A version of this story appeared in
Volume 102, Issue 28
Connecting a trifluoromethyl group to an organic compound through a heteroatom such as nitrogen, oxygen, or sulfur can help chemists get the properties they want when designing small-molecule drugs or agrochemicals. But the leading methods for making those connections tend to rely on reagents that are expensive, atom inefficient, and difficult to scale up.
Timothy Noël and his team at the University of Amsterdam wanted to fix that. They developed a flow-chemistry approach to making trifluoromethylated nitrogen, oxygen, and sulfur anions from widely available precursors and attaching them to organic molecules (Science 2024, DOI: 10.1126/science.adq2954). Because the method creates the reactive fluorinated species as needed, Noël says, it cuts down on waste. “You can just make it on demand in your environment, and you’re ready to go.”
It also doesn’t use any reagents that could be considered per- and polyfluoroalkyl substances (PFAS) under proposed European Union regulations that would tightly restrict their use. Noël says this wasn’t something he and his team were specifically aiming for, but it’s “a nice collateral effect.”
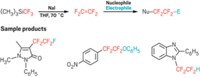
The researchers started with commercially available or easy-to-make chlorinated precursors and passed them through a packed column of cesium fluoride to generate reactive trifluoromethylated anions. They then channeled the anions directly into nucleophilic substitution reactions to furnish a wide range of organic products bearing trifluoromethylated nitrogen, oxygen, or sulfur atoms. Collaborators at AstraZeneca provided the team with derivatives of drug molecules to test the new method on and make sure it would be suitable for late-stage functionalization.
The chlorinated precursors are derivatives of phosgene, a toxic gas used to manufacture plastics and pesticides, but Noël says there’s little need for alarm. These chemicals are liquids and already used in process chemistry, so chemists already know how to mitigate the risks they carry. The flow system makes it easier to manage them safely.
Fluorine chemist Tobias Ritter of the Max Planck Institute for Kohlenforschung, who was not involved in the work, calls it “an elegant solution to access valuable molecules.” He adds that it’s a great example of using flow chemistry to streamline tricky synthesis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter