Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Fluorination
A cheap, flexible fluoroalkylation
Scientists invent a versatile method for attaching fluorinated groups to alkenes using inexpensive feedstocks and Earth-abundant catalysts
by Brianna Barbu
November 14, 2023
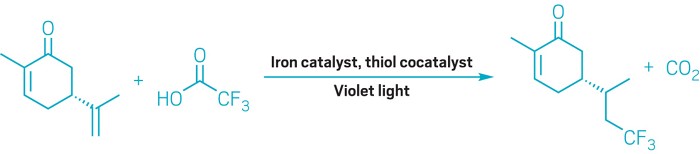
A new reaction that uses commercially available fluoroalkyl carboxylic acids and Earth-abundant catalysts could provide a more economic and versatile way to add fluorinated groups to molecules (Nat. Chem. 2023 DOI: 10.1038/s41557-023-01365-0).
“We want everything to be basically dirt cheap,” says Julian West of Rice University, who led the work.
Fluoroalkyl groups can have big benefits for drug molecules’ stability and behavior in the body. Scientists had been eyeing trifluoroacetic acid and its cousins as cheap sources of fluoroalkyl groups for a while. But a longstanding challenge was finding a way to use them without resorting to harsh conditions or fancy catalysts to overcome the molecules’ high oxidation potentials.
In this new paper, West and coworkers describe how they overcame that challenge by using an iron photocatalyst that coordinates to the carboxylate and sneakily takes an electron from it through an inner-sphere process. The acid then loses carbon dioxide to give a fluoroalkyl radical that happily attaches to electron-rich alkenes. Finally, a thiol cocatalyst furnishes the product with a hydrogen atom and completes the catalytic cycle by re-oxidizing the iron.
Because the reaction takes place under mild conditions and tolerates a wide variety of functional groups on the alkene, it can be used for late-stage functionalization. The researchers demonstrated this by using their new technique to add trifluoromethyl and other fluoroalkyl groups to complex molecules such as drugs and natural products. West says his grad students “really wanted to try to break the method. So they just kept trying more complicated molecules, and it kept working.”
In an email, Jie Wu at the National University of Singapore calls the paper a “significant breakthrough” and praises it for making use of inexpensive catalysts to unlock the synthetic potential of economical fluorine sources.
Dani Schultz, a process chemist at Merck & Co. who was not involved in the work, calls it “a clever solution to a long-standing problem” and says she expects that the method will quickly be adopted for drug discovery. Chemists could easily use it to create multiple variants of a molecule with different fluorinated groups and compare their activities, she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter