Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Birch reduction runs ammonia-free
Crown ethers simplify the set-up of classic reaction
by Tien Nguyen
June 17, 2018
| A version of this story appeared in
Volume 96, Issue 25

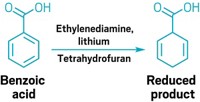
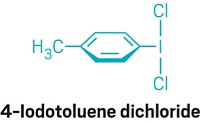
To break compounds’ aromaticity, chemists commonly rely on the 74-year-old Birch reduction, which uses sodium metal and ammonia. However, the components’ moisture-sensitive nature—requiring practitioners to trot out potentially hazardous ammonia tanks to freshly distill the reagent—makes running the reaction a chore. A modified Birch reduction developed in Jie An’s lab at China Agricultural University lets scientists skip the arduous set-up (Org. Lett. 2018, DOI: 10.1021/acs.orglett.8b00891). The researchers’ first experiences with the Birch reduction, attempting to reduce graphite to graphene, resulted in long set-up times and a small, but likely still smelly, ammonia leak, spurring them to try to simplify things. The new method replaces ammonia with the commercial crown ether 15-crown-5, which can be recovered after the reaction. The crown ether takes on ammonia’s role for the most part; it mixes with a bench-stable sodium dispersion to form the solvated electrons that reduce the substrates and give the reaction its signature blue hue. However, tertiary amides, typically converted to alcohols under standard Birch conditions, survived in the team’s reaction, suggesting that the crown ether reagent switches the mechanism from an inner- to an outer-sphere electron transfer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter