Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Cellulose derivative accelerates organic chemistry in water
Additive facilitates numerous pharmaceutically relevant reactions through unknown mechanism
by Carmen Drahl
June 24, 2018
| A version of this story appeared in
Volume 96, Issue 26

To reduce the environmental impact of the waste they create, chemists would like to replace organic solvents with water. New additives derived from cellulose—a biodegradable material from plants—may help. First reported at the Green Chemistry & Engineering Conference in Portland, Ore., last week, the additives accelerate aqueous versions of reactions commonly run by medicinal chemists.
Chemists already have developed surfactants that create tiny compartments in water so that normally insoluble organic chemicals can react. Though the surfactants are effective, the reactions still can take hours to reach completion.
In Portland, Wilfried Braje of the pharmaceutical company AbbVie reported a Buchwald-Hartwig amination that reached 95% yield in water in five minutes, thanks to the inclusion of 2% by weight of a cellulose derivative. The same reaction run with an established surfactant took three hours to achieve a comparable yield, while the amination run in organic solvent required as many as 72 hours, and higher temperatures, to do so.
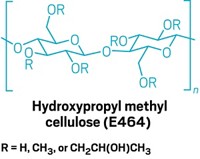
A patent (WO 2017129796A1) filed by AbbVie names the additive in that reaction as hydroxypropyl methylcellulose and demonstrates the technology’s effectiveness in amide couplings, C–H activations, and other reactions. Braje declined to comment for this article beyond checking it for factual accuracy in accordance with the embargo policy of the journal where his team plans to submit the work.
AbbVie’s technology “has great potential,” says Sachin Handa of the University of Louisville, who has independently reported cellulose-palladium nanoparticles for cross-coupling chemistry. How the cellulose derivatives work is unclear, but he notes that cellulose has a chiral surface with free hydroxyl groups that could act as hydrogen bond donors or acceptors. Because of this chemistry, he says cellulose derivatives could affect the speeds and outcomes of many chemical transformations.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter